Abstract
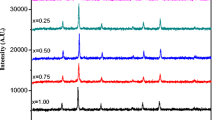
Magnesium ferrite (MgFe2O4) was prepared by simple low-cost oxalate coprecipitation method and characterized by XRD and SEM. The X-ray analysis confirmed the formation of a single-phase cubic spinel structure. The product revealed a non-uniform morphology and some certain extent of agglomeration. Crystallite size, texture coefficient, dislocation density, hopping length, and mechanical properties of the product are also reported.
Similar content being viewed by others
References
Sanpo, N., Solution precursor plasma spray system, in Springer Briefs in Materials, 2014, pp. 5–50. http://www.springer.com/978-3-319-07024-7
Goldman, A., Modern Ferrite Technology, New York: Springer, 2006. http://www.springer.com/la/book/9780387281513
Vinayak, V., Khirade, P.P., Birajdar, S.D., Gaikwad, P.K., Shinde, N.D., and Jadhav, K.M., Low temperature synthesis of magnesium doped cobalt ferrite nanoparticles and their structural properties, Int. Adv. Res. J. Sci. Eng. Technol., 2015, vol. 2, no. 3, pp. 55–58. doi 10.17148/IARJSET.2015.2313
Maensiri, S., Masingboon, C., Boonchom, B., and Seraphin, S., A simple route to synthesize nickel ferrite (NiFe2O4) nanoparticles using egg white, Scr. Mater., 2007, vol. 56, no. 9, pp. 797–800. http://dx.doi.org/doi 10.1016/j.scriptamat.2006.09.033
Waqus, H. and Quresghi, A.H., Influence of pH on nanosized Mn–Zn ferrite synthesized by sol–gel auto combustion process, J. Therm. Anal. Calorim., 2009, vol. 98, no. 2, pp. 355–360. doi 10.1007/s10973-009-0289-8
Singhal, S., Singh, J., Barthwal, S.K., and Chandra, K., Preparation and characterization of nanosize nickelsubstituted cobalt ferrites (CoNiFeO), J. Solid State Chem., 2005, vol. 178, no. 10, pp. 3183–3189. http://dx.doi.org/doi 10.1016/j.jssc.2005.07.020
Prinetto, F., Tichit, D., Teissier, R., and Coq, B., Mgand Ni-containing layered double hydroxides as soda substitutes in the aldol condensation of acetone, Catal. Today, 2000, vol. 55, no. 1–2, pp. 103–116. http://dx.doi.org/doi 10.1016/S0920-5861(99)00230-8
Murcia-Mascaros, S., Navarrao, R., Gomez-Sainero, L., Costantio, U., Nocchetti, J.L., and Fierro., G., Oxidative methanol reforming reactions on CuZnAl catalysts derived from hydrotalcite-like precursors, J. Catal., 2001, vol. 198, no. 2, pp. 338–347. doi 10.1006/jcat.2000.3140
Rane, K.S., Verenkar, V.M.S., and Sawant, P.Y., Dielectric behavior of MgFe2O4 prepared from chemically beneficiated iron ore rejects, Bull. Mater. Sci., 2008, vol. 24, no. 3, pp. 323–330. doi 10.1007/BF02704930
Liu, Y.L., Liu, Z.M., Yang, Y., Yang, H.F., Shen, G.L., and Yu, R.Q., Simple synthesis of MgFe2O4 nanoparticles as gas sensing materials, Sens. Actuators B, 2005, vol. 107, no. 2, pp. 600–604. doi 10.1016/j.snb. 2004.11.026
Candeia, R.A., Souza, M.A.F., Bernardi, M.I.B., Maestrelli, S.C., Santos, I.M.G., Souza, A.G., and Longo, E., MgFe2O4 pigment obtained at low temperature, Mater. Res. Bull., 2006, vol. 41, no. 1, pp. 183–190.190. doi 10.1016/j.materresbull.2005.07.019
Dayakar, T., Venkateswara Rao, K., and Shilpa Chakra, Ch., Synthesis and characterization of MgFe2O4(0.5)/TiO2(0.5) nano ceramic pigment by mechanochemical synthesis, Int. J. Nano Sci. Technol., 2013, vol. 1, no. 1, pp. 1–8. http://www.ijnst.com/
Sepelak, V., Bergmann, I., Feldhoff, M.A., Litterst, F.J., Becker, K.D., Cadogan, J.M., Hofmann, M., Hoelzel, M., Wang, J.L., Avdeev, M., and Campbell, S.J., Mechanosynthesis of nanocrystalline MgFe2O4: Neutron diffraction and Mössbauer spectroscopy, Hyperfine Interact., 2010, vol. 198, no. 1, pp. 67–71. doi 10.1007/s10751-010-0243-y
Berchmans, L.J., Selvan, R.K., Kumar, P.N.S., and Augustin, C.O., Structural and electrical properties of Ni1–xMgxFe2O4 synthesized by citrate gel process, J. Magn. Magn. Mater., 2004, vol. 279, no. 23, pp. 103–110. doi 10.1016/j.jmmm.2004.01.073
Iqbal, M.J., Ahmad, Z., Melikhov, Y., and Niebedim, I.C., Temperature and composition dependence of magnetic properties of cobalt–chromium co-substituted magnesium ferrite nanomaterials, J. Magn. Magn. Mater., 2012, vol. 324, no. 23, pp. 3986–3990. http://dx.doi.org/doi 10.1016/j.jmmm.2012.06.031
Akhtar, M.J. and Younas, M., Structural and transport properties of nanocrystalline MnFe2O4 synthesized by coprecipitation method, Solid State Sci., 2012, vol. 14, no. 10, pp. 1536–1542. http://dx.doi.org/doi 10.1016/j.solidstatesciences.2012.08.026
Yattinahalli, S.S., Kapatkar, S.B., and Mathad, S.N., Structural and mechanical properties of a nano ferrite, Adv. Sci. Focus, 2014, vol. 2, no. 1, pp. 42–46. doi 10.1166/asfo.2014.1079
Khot, V.M., Salunkhe, A.B., Phadatare, M.R., and Pawar, S.H., Formation, microstructure, and magnetic properties of nanocrystalline MgFe2O4, Mater. Chem. Phys., 2012, vol. 132, no. 2, pp. 782–787. http://dx.doi.org/doi 10.1016/j.matchemphys.2011.12.012
Mathad, S.N. and Puri, V., Microwave studies of environmental friendly ferroelectrics, Int. Schol. Res. Notices, 2014, vol. 2014, article ID 683986. http://dx.doi.org/doi 10.1155/2014/683986
Mathad, S.N., Jadhav, R.N., Patil, N.D., and Puri, V., Structural and mechanical properties of Sr+2-doped bismuth manganite thick films, Int. J. Self-Propag. High-Temp. Synth., 2013, vol. 22, no. 4, pp. 180–184. doi 10.3103/S1061386213040018
Mathad, S.N., Jadhav, R.N., Phadatare, V., and Puri, V., Structural and mechanical properties of Sr-doped barium niobate thick films, Int. J. Self-Propag. High-Temp. Synth., 2014, vol. 23, no. 3, pp. 145–150. doi 10.3103/S106138621403008X
Pathan, A.T., Mathad, S.N., and Shaikh, A.M., Infrared spectral studies of Co2+-substituted LiNi–Zn nano-structured ferrites, Int. J. Self-Propag. High-Temp. Synth., 2014, vol. 23, no. 2, pp. 112–117. doi 10.3103/S1061386214020083
Wurst, J.C. and Nelson, J.A., Lineal intercept technique for measuring grain size in two-phase polycrystalline ceramics. J. Am. Ceram. Soc. Bull., 1972, vol. 55, no. 1, pp. 109–111. doi 10.1111/j.1151-2916.1972.tb11224.x
Suryawanshi, S.S., Deshpand, V., and Sawanth,S.R, J. Mater. Chem. Phys., 1999, vol. 59, no. 2, pp. 199–203. doi 10.1016/S0254-0584(99)00046-2
Raghasudha, M., Ravinder, D., and Veerasomaiah, P., Characterization of nano-structured magnesium chromium ferrites synthesized by citrate–gel autocombustion method, Adv. Mater. Lett., 2013, vol. 4, no. 12, pp. 910–916. doi 10.5185/amlett.2013.5479
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Shedam, R.M., Gadkari, A.B., Mathad, S.N. et al. Structural and mechanical properties of nanograined magnesium ferrite produced by oxalate coprecipitation method. Int. J Self-Propag. High-Temp. Synth. 26, 75–79 (2017). https://doi.org/10.3103/S1061386217010113
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386217010113