Abstract
An elevated level of circulatory interleukin 6 (IL-6) is a biomarker for cytokine storm of various etiologies, including COVID-19, and contributes to poor prognosis. Vascular endothelial cells are one of the main targets of pathological action of IL-6. IL-6 activates the trans-signaling pathway via the formation of the IL-6/sIL-6Ra/gp130 receptor complex and subsequent activation of the JAK/STAT3 signaling pathway and PI3K/AKT and MEK/ERK kinases in some cases. Previously, it was shown by the authors' group and other researchers that reactive oxygen species (ROS), including mitochondrial ROS (mito-ROS), contribute to the induction of IL-6 expression in the endothelium, mainly due to increased activation of the transcription factor NF-kB. We have also shown that the mitochondria-targeted antioxidant SkQ1 (Plastoquinolyl-10(6'-decyltriphenyl)phosphonium) prevented tumor necrosis factor (TNF)-induced cytokine storm and death in mice. In the aortas of these animals, SkQ1 also prevented the increase in the expression of NF-kB-dependent genes, including the cytokine IL-6 and the chemokine MCP-1. In the current work, the hypothesis of mito-ROS involvement in the IL-6-signaling-mediated proinflammatory gene expression in endothelial cells is tested. SkQ1 suppressed the expression and secretion of the MCP-1 chemokine, induced by IL-6 in combination with sIL-6-Ra, but not the expression of ICAM-1 adhesion molecules in EA.hy926 human endothelial cells. Using specific inhibitors, the authors have shown that, in EA.hy926 cells, IL-6-induced expression of MCP-1 and ICAM-1 depends on the signaling protein and transcription activator STAT3 and, in some cases, on JNK, PI3K, and MEK1/2 kinases and is independent of p38 kinase. In this model, IL-6 induced rapid STAT3 activation, while ERK1/2 activation was less pronounced, and there was no IL-6 effect on Akt and JNK activation. SkQ1 partially suppressed STAT3 and ERK1/2 activation. Thus, we have shown that SkQ1 suppresses not only NF-kB-dependent expression of IL-6 and other proinflammatory genes but also IL-6-induced activation of JAK/STAT3 and STAT3-dependent expression of MCP-1, which probably contributes to the overall therapeutic effect of SkQ1.
Similar content being viewed by others
INTRODUCTION
Interleukin-6 (IL-6) is a multifunctional pleiotropic cytokine, the main source of which is monocytes and macrophages and which can be secreted by many other cell types. A constant increase in the level of IL-6 accompanies the development of cardiovascular diseases as well as chronic inflammatory, autoimmune, and oncological diseases [1, 2]. In acute systemic inflammation, excessive production of IL-6 is a biomarker of cytokine storm [3]. Cytokine storm is an excessive unbalanced immune response that can result from infection with pathogenic bacteria or viruses or be noninfectious in nature, for example, trauma, ischemia, graft versus host disease, autoimmune diseases, consequences of engineered T-cell therapy in patients with leukemia, etc. [4]. During a cytokine storm, a large number of various active inflammatory mediators, such as cytokines, chemokines, and some growth factors, are produced, which contributes to the progression of concomitant diseases [4]. IL-6 occupies a central place Among cytokines, the level of which is elevated in patients with a poor prognosis of COVID-19 [5]. An elevated level of IL-6 is a predictor of severe course and death in COVID-19 [6–8].
To date, both the blockade of IL-6 itself and the suppression of IL-6R signaling are used in the treatment of a number of chronic inflammatory diseases [3]. Numerous clinical trials are currently underway to evaluate the effectiveness of tocilizumab (humanized antibodies against IL-6R) for the treatment of complications of COVID-19, but the results of these trials are very controversial [9].
Among the targets of IL-6, a special place is occupied by the vascular endothelium, which regulates the permeability of blood vessels and the migration of cells from blood to tissues, and is also involved in the regulation of blood coagulation and vascular tone. IL-6 acts on endothelial cells by forming a complex with the soluble form of the receptor (sIL-6R) and receptor glycoprotein 130 (gp130 or CD130), which is expressed in all cell types.
Receptor complexes with gp130 usually activate the JAK/STAT signaling pathway, but PI3K/AKT or MEK/ERK can also be activated in different cell types [1, 3]. It has been reported that IL-6 causes an increase in the permeability of the vascular endothelium [10, 11] and an increase in the expression of adhesion molecules ICAM-1, VCAM-1, and E-selectin, as well as the cytokine IL-6 and a number of chemokines: CXCL10/IP-10, CCL4/MIP-1β, CCL5/RANTES, CCL11/Eotaxin-1, CCL17/TARC, CCL2/MCP-1, and CXCL8/IL-8, which promotes leukocyte infiltration of organs [12–16]. It has also been shown that IL-6 stimulates the expression of PAI-1 (plasminogen activator inhibitor-1; inhibitor of plasminogen activator-1), which enhances coagulation [17]. Abnormal activation of the endothelium leads to its dysfunction, which is directly related to the development of cardiovascular and metabolic diseases, and is also a frequent complication of cytokine storms [18, 19]. In COVID-19, profound endothelial dysfunction and vascular endothelial injury is the main cause of both acute respiratory distress syndrome (ARDS) and extrapulmonary complications, such as acute myocardial injury, renal failure, or thromboembolic complications [20–23].
Reactive oxygen species (ROS), including those produced by mitochondria (mito-ROS), play an important role in the physiology and pathophysiology of the vascular system [23–25]. ROS are involved in the production of IL-6 in response to cytokines (TNF, IL-1b, IL-4), angiotensin-2, and also under hypoxic conditions [26–30]. We have previously shown that a decrease in the level of mito-ROS with the help of the mitochondria-targeted antioxidant SkQ1 (10-(6'-Plastoquinonyl)decyltriphenylphosphonium) leads to the suppression of TNF-stimulated IL-6 secretion and the expression of a number of proinflammatory genes in cultured endothelial cells as well as expression of a number of proinflammatory genes, including IL-6 in the aortas of mice injected with lethal doses of TNF [31, 32]. Moreover, SkQ1 prevented the death of these animals [32]. The action of the antioxidant was mainly associated with associated with the suppression of the activation of the transcription factor NF-kB, which regulates the expression of many proinflammatory genes, including IL-6. At the same time, it was reported that IL-6 itself can induce ROS generation, which contributes to the development of endothelial dysfunction [15, 33]. However, there are very few such studies, and nothing is known about the involvement of mito-ROS in IL-6 trans-signaling. In the present work, we assessed the possible involvement of mito-ROS in IL-6-induced expression of proinflammatory genes in endothelial cells using SkQ1.
MATERIALS AND METHODS
Materials. SkQ1 was synthesized by G.A. Korshunova and N.V. Sumbatyan at the Belozersky Research Institute of Physical and Chemical Biology. Reagents produced by Sigma (United States) and culture plastic produced by Costar (United States) were used in the work, except when otherwise indicated.
Cells and scheme of experiments. Human endothelial cells of the EA.hy926 line (ATCC CRL-2922) were grown in DMEM medium with 4.5 g/L glucose (PanEco, Russia) containing 10% fetal calf serum (Hyclone, United States), 100 µM hypoxanthine, and 20 µM thymidine (PanEco, Russia). Antioxidants (100 μM Trolox, 20 nM SkQ1) were added to cells placed in 12-well plates (100 000 cells per well) after attachment and spreading. After 4 days, the medium was changed to a new one containing 0.2% fetal calf serum, and antioxidants were added again. After 12–15 h, recombinant human IL-6 (GenScript, United States) mixed with sIL-6Ra (GenScript, United States) (concentrations and exposure times are indicated in the figure captions) was added. Inhibitors of STAT-3 (Stattic, 10 µM; Apex Bio, United States), JNK (SP600125, 20 µM; Enzo, United States), PI3K (LY294002, 10 µM; Cell Signaling, United States), and MEK1/2 (UO126, 10 µM; Cell Signaling, United States) and p38 (SB203580, 5 μM; Cell Signaling, United States) were added 15 min before the mixture of IL-6 with sIL-6Ra.
Determining MCP-1 concentration in the growth medium. The concentration of MCP-1 in the growth medium was determined using the MCP-1-ELISA-BEST kit (Vector-BEST, Russia) according to the manufacturer’s protocol.
RNA isolation, reverse transcription and real-time polymerase chain reaction (PCR). Total RNA was isolated from cultured cells using an RNA isolation kit (Zymo Research; Quick-RNA MiniPrep, United States) according to the manufacturer’s protocol. DNA was destroyed by treating the samples with DNase (Thermo Fisher Scientific, United States), RNA was precipitated with alcohol, washed, dried, and dissolved in water. cDNA was obtained using the RevertAid RT Kit (Thermo Fisher Scientific, United States) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using the PCR-Mix reaction mixture with EVA Green and ROX (Synthol, Russia).
cDNA was obtained using the RevertAid RT Kit (Thermo Fisher Scientific, United States) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using the PCR-Mix reaction mixture containing EVA Green and ROX (Synthol, Russia). The reaction was carried out in an iCycler iQ amplifier (Bio-Rad, United States) under the following conditions: 95°C for 3 min. → (94°C for 15s → 56°C for 20s →72°C for 20s) ×40. Relative gene expression was calculated using the 2-ΔΔCt quantification method. The mRNA expression values of the target genes were normalized to the expression values of the reference RPL32 gene. Primer sequences are listed in the Table 1.
Western blot. Immunoblotting was performed as described previously [31]. To visualize the total protein, 0.5% (V/V) 2,2,2-Trichloroethanol was incorporated into the gel [34]. Antibodies against the following human proteins were used: STAT3 (#CB10245, Cell Applications, United States) and phospho-STAT3 (Tyr705; #MA5-15193; Invitrogen, United States), Akt (#2967), phospho-Akt (Ser473; #4051), ERK1/2 (#4695), phospho-ERK1/2 (Thr202/Tyr204; #4370), p38 (#4631), phospho-p38 (Thr180/Tyr182; #9212), SAPK/JNK (#9258), phospho-SAPK/JNK (Thr183/Tyr185; #4668S; Cell Signaling, United States), as well as horseradish peroxidase-labeled antibodies against rabbit or mouse immunoglobulins. To visualize the peroxidase reaction, the SuperSignal West Dura kit (Thermo Fisher Scientific, United States) was used in accordance with the manufacturer’s protocol. Images were obtained using the ChemiDoc™ MP System (Bio-Rad, United States). The obtained images were analyzed using ImageLab software (version 5.2.1, Bio-Rad, United States).
Statistical processing. Data are presented as mean ± standard deviation. Groups were compared using two-way analysis of variance (ANOVA, Sidak multiple comparison test) using GraphPad Prism 6 software (GraphPad Software, United States).
RESULTS AND DISCUSSION
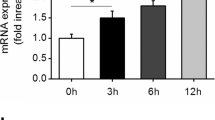
IL-6 (400 ng/mL) in combination with sIL-6Ra (400 ng/mL) significantly stimulated the expression of MCP-1, ICAM-1, and IL-6 (Fig. 1a) in human endothelial cells of the EA.hy926 line. Peak expression of these proinflammatory genes was observed 1 h after the addition of IL-6 in combination with sIL-6Ra. We also observed an increase in the level of MCP-1 in the growth medium of EA.hy926 cells after the addition of IL-6 together with sIL-6Ra (Fig. 1b). The peak of MCP-1 secretion occurred 8 h after the addition of IL-6 in combination with sIL-6Ra.
Antioxidants SkQ1 and Trolox inhibit IL-6-induced MCP-1 mRNA expression and secretion of this chemokine. Cells were treated with SkQ1 (20 nM) and Trolox (100 µM) antioxidants; after 4 days, a mixture of IL-6 with sIL-6Ra (400 ng/mL) was added. Expression of mRNA of proinflammatory genes was determined 1 h later and MCP-1 secretion 8 h after the addition of a mixture of IL-6 with sIL-6Ra. (a) Effect of IL-6 on mRNA expression of proinflammatory genes in EA.hy926 cells. (b–d) Effect of antioxidants SkQ1 (20 nM, 4 days) and Trolox (100 μM, 4 days) on (b) IL-6-induced secretion of MCP-1 and (c) expression of MCP-1 mRNA and (d) ICAM-1 mRNA. N ≥ 4, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 compared to treated with IL-6 alone with sIL-6Ra cells.
An increase in the expression of PAI-1, IL-8, and RANTES mRNA under the action of IL-6 in combination with sIL-6Ra was not noted by us in EA.hy926 cells (Fig. 1a). Obviously, this is due to the fact that the EA.hy926 cells lack some of their endothelial properties.
The mitochondria-targeted antioxidant SkQ1 and the classical antioxidant Trolox (a water-soluble analogue of vitamin E) statistically significantly suppressed the increase in the content of MCP-1 in the growth medium and the increase in the expression of MCP-1 mRNA caused by the addition of IL-6 in combination with sIL-6Ra (Figs. 1b, 1c). No significant suppression of ICAM-1 expression was observed in this model, which indicates the existence of differences in the regulation of the expression of these genes (Fig. 1d).
Using chemical inhibitors, we evaluated the contribution of JAK/STAT, PI3K/AKT, and MEK/ERK signaling pathways to IL-6-induced stimulation of MCP-1 and ICAM-1 mRNA expression together with sIL-6Ra in EA.hy926 cells (Figs. 2a, 2b). The expression of MCP-1 and ICAM-1 was suppressed to the greatest extent by the STAT3 inhibitor. The effect of JNK, PI3K, and MEK1/2 inhibitors was less pronounced. The p38 inhibitor did not suppress the expression of MCP-1 and ICAM-1.
Decreased expression of MCP-1 mRNA under the action of the antioxidant SkQ1 is at least partly due to its ability to suppress STAT3 activation. Cells were treated with antioxidants and a mixture of IL-6 with sIL-6Ra, as indicated in the caption to Fig. 1. Inhibitors were added 15 min before the IL-6/sIL-6Ra mixture. Samples for PCR were collected 1 h later; those for Western blots: 7–60 min after the addition of a mixture of IL-6 with sIL-6Ra. (a, b) Effect of STAT-3 (static, 10 µM), JNK (SP600125, 20 µM), PI3K (LY294002, 10 µM), and MEK1/2 (UO126, 10 µM) and p38 (SB203580, 5 µM) inhibitors on induced IL-6 with sIL-6Ra mRNA expression of (a) MCP-1 and (b) ICAM-1. (c, d) Effect of SkQ1 (20 nM, 4 days) and Trolox (100 µM, 4 days) on the activation (phosphorylation) of STAT3, ERK1/2, Akt, and SAPK/JNK under the action of IL-6 with sIL-6Ra (7–60 min). (c) Images of typical Western blots; (d) densitometric analysis for Western blots. N ≥ 3, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 compared to treated with IL-6 alone with sIL-6Ra cells.
Under our experimental conditions on endothelial cells of the EA.hy926 line, IL-6, together with sIL-6Ra, caused a rapid and pronounced activation of STAT3 (phosphorylation of Tyr705) and a less noticeable activation of ERK1/2 (phosphorylation of Thr202/Tyr204) but did not affect the activation of Akt-1 (phosphorylation of Ser473) and SAPK/JNK (phosphorylation of Thr183/Tyr185) (Figs. 2c, 2d). SkQ1 partially suppressed STAT3 phosphorylation and, to a lesser extent, ERK1/2 phosphorylation (Figs. 2c, 2d). Thus, mito-ROS enhance the expression of MCP-1 mRNA to a large extent by increasing the activation of STAT3.
IL-6 is an important inflammatory mediator which elevated level is associated with cardiovascular and chronic inflammatory diseases as well as cytokine storms of various etiologies and their poor outcomes [2, 3]. ROS, including mito-ROS, increase the expression of IL-6, which contributes to increased inflammation and the development of pathologies [26–30]. There are also few reports on the involvement of ROS in the transmission of inflammatory signals from the IL-6-sIL-6Ra-gp130 receptor complex [15, 33]. It has been shown that IL-6 is able to induce oxidative stress in cultured vascular smooth muscle cells and mouse vessels by stimulating the expression of the angiotensin II receptor type 1 [33]. Resveratrol (a red wine polyphenol with antioxidant properties) has also been reported to suppress IL-6-induced Rac1-dependent ROS generation and ICAM-1 expression in the endothelium [15]. We have shown that the mitochondria-targeted antioxidant SkQ1 and the classical antioxidant Trolox equally suppress IL-6-sIL-6-R1-induced mRNA expression and secretion of the MCP-1 protein (Figs. 1b, 1c). This indicates the involvement of mito-ROS in the transmission of the inflammatory signal from the IL-6-sIL-6Ra-gp130 receptor complex in endothelial cells.
Using chemical inhibitors, we have shown that expression of MCP-1 in EA.hy926 cells, stimulated by IL-6 in combination with sIL-6Ra, depends on STAT3 and, to a lesser extent, on PI3K and MEK1/2 as well as on JNK but not on p38 (Fig. 2a), which corresponded to the data obtained on other endothelial cultures [1, 3]. In endothelial cells of the EA.hy926 line, IL-6, together with sIL-6Ra, caused a rapid and pronounced activation of STAT3 and a slight activation of ERK1/2 but did not affect the activation of Akt-1 and JNK (Fig. 2c). We also showed that SkQ1 suppresses STAT3 and, to a lesser extent, ERK1/2 activation (Figs. 2c, 2d). Thus, in the model used, the mechanism of the anti-inflammatory action of SkQ1 was largely associated with the suppression of STAT3 activation.
Previously, resveratrol was shown on to suppress IL-6-induced ICAM-1 expression by preventing STAT3 phosphorylation in bovine aortic endothelium [15]. Analysis using chemical inhibitors revealed the same major expression regulators for MCP-1 and ICAM-1 in our model (Figs. 2a, 2b). However, to our surprise, neither Trolox nor SkQ1 affected IL-6-induced expression of ICAM-1 mRNA in EA.hy926 cells (Fig. 1d). This indicates the existence of differences in the redox regulation of the expression of these genes in different species.
Antioxidants, including mitochondria-targeted ones, showed a pronounced therapeutic effect in some models of sepsis and systemic inflammatory response syndrome in animals [32, 35, 36]. However the antioxidants SkQ1 and mito-Tempo were ineffective in a model of sepsis caused by ligation and puncture of the caecum in mice [37]. We have previously shown that SkQ1 prevents the temperature drop and death of mice injected with lethal doses of TNF and suppresses the expression of NF-kB regulated genes (VCAM-1, ICAM-1, MCP-1, and IL-6) in the aortas of these mice. [32].
In endothelial cells in vitro, SkQ1 suppressed TNF-stimulated activation of the transcription factor NF-kB and, as a consequence, NF-kB-dependent expression of ICAM-1, VCAM-1, E-selectin, and MMP-9 as well as the secretion of IL-6 and IL-8 [31, 32]. Using transgenic mice, it has been shown that suppression of NF-kB transcription factor activation in the endothelium prevents endothelial dysfunction in models of lipopolysaccharide-induced sepsis or by caecal ligation and puncture [38].
However, the therapeutic effect of SkQ1 does not appear to be limited to inhibition of NF-kB activation. SkQ1 has a complex angioprotective effect on the vascular endothelium: it suppresses TNF-induced adhesion of HL-60 promyelocytic leukemia cells to the endothelium [31], disassembly of intercellular contacts, and an increase in the permeability of the endothelial barrier [32, 39, 40] as well as apoptosis of endothelial cells [41]. In the first two cases, the action of SkQ1 was largely associated with the prevention of NF-kB activation, while the suppression of apoptosis and caspase-dependent destruction of intercellular contacts and endothelial permeability was associated with the prevention of the release of cytochrome c from mitochondria. In addition, SkQ1 inhibited mast cell degranulation in vivo and in vitro, which could also contribute to a decrease in endothelial activation during acute inflammation [42, 43]. We have now supplemented this picture with the data that SkQ1 suppresses not only NF-kB-dependent expression and secretion of IL-6 but also IL-6-induced activation of JAK/STAT3 and, as a consequence, expression of MCP-1, which may contribute to the overall therapeutic effect of SkQ1.
REFERENCES
Uciechowski, P. and Dempke, W.C.M., Interleukin-6: A masterplayer in the cytokine network, Oncology, 2020, vol. 98, no. 3, pp. 131–137.
Hou, T., Tieu, B.C., Ray, S., Recinos III, A., Cui, R., Tilton, R.G., and Brasier, A.R., Roles of IL-6-gp130 signaling in vascular inflammation, Curr. Cardiol. Rev., 2008, vol. 4, no. 3, pp. 179–192.
Kang, S. and Kishimoto, T., Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms, Exp. Mol. Med., 2021, vol. 53, no. 7, pp. 1116–1123.
Fajgenbaum, D.C. and June, C.H., Cytokine storm, N. Engl. J. Med., 2020, vol. 383, no. 23, pp. 2255–2273.
Liu, Y., Chen, D., Hou, J., Li, H., Cao, D., Guo, M., Ling, Y., Gao, M., Zhou, Y., Wan, Y., and Zhu, Z., An inter-correlated cytokine network identified at the center of cytokine storm predicted COVID-19 prognosis, Cytokine, 2021, vol. 138, p. 155365.
Chen, G., Wu, D.I., Guo, W., et al., Clinical and immunological features of severe and moderate coronavirus disease 2019, J. Clin. Invest., 2020, vol. 130, no. 5, pp. 2620–2629.
Herold, T., Jurinovic, V., Arnreich, C., Lipworth, B.J., Hellmuth, J.C., von Bergwelt-Baildon, M., Klein, M., and Weinberger, T., Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J. Allergy Clin. Immunol., 2020, vol. 146, no. 1, pp. 128–136.e4.
Ruan, Q., Yang, K., Wang, W., Jiang, L., and Song, J., Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med., 2020, vol. 46, no. 5, pp. 846–848.
Ascierto, P.A., Fu, B., and Wei, H., IL-6 modulation for COVID-19: the right patients at the right time?, J. Immunother. Cancer, 2021, vol. 9, no. 4, p. e002285.
Alsaffar H., Martino N., Garrett J.P., Adam A.P. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis, Am. J. Physiol.: Cell Physiol., 2018, vol. 314, no. 5, pp. C589–C602.
Desai, T.R., Leeper, N.J., Hynes, K.L., and Gewertz, B.L., Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway, J. Surg. Res., 2002, vol. 104, no. 2, pp. 118–123.
Watson, C., Whittaker, S., Smith, N., Vora, A.J., Dumonde, D.C., and Brown, K.A., IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes, Clin. Exp. Immunol., 1996, vol. 105, no. 1, pp. 112–119.
Romano, M., Sironi, M., Toniatti, C., Polentarutti, N., Fruscella, P., Ghezzi, P., Faggioni, R., Luini, W., Van Hinsbergh, V., Sozzani, S., Bussolino, F., Poli, V., Ciliberto, G., and Mantovani, A., Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment, Immunity, 1997, vol. 6, no. 3, pp. 315–325.
Wung, B.S., Ni, C.W., and Wang, D.L., ICAM-1 induction by TNFα and IL-6 is mediated by distinct pathways via Rac in endothelial cells, J. Biomed. Sci., 2005, vol. 12, no. 1, pp. 91–101.
Wung, B.S., Hsu, M.C., Wu, C.C., and Hsieh, C.W., Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: Effects on the inhibition of STAT3 phosphorylation, Life Sci., 2005, vol. 78, no. 4, pp. 389–397.
McLoughlin, R.M., Jenkins, B.J., Grail, D., Williams, A.S., Fielding, C.A., Parker, C.R., Ernst, M., Topley, N., and Jones, S.A., IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation, Proc. Natl. Acad. Sci. U. S. A., 2005, vol. 102, no. 27, pp. 9589–9594.
Kang, S., Tanaka, T., Inoue, H., Ono, C., Hashimoto, S., Kioi, Y., Matsumoto, H., Matsuura, H., Matsubara, T., Shimizu, K., and Ogura, H., IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome, Proc. Natl. Acad. Sci. U. S. A., 2020, vol. 117, no. 36, pp. 22351–22356.
Xu, S., Ilyas, I., Little, P.J., Li, H., Kamato, D., Zheng, X., Luo, S., Li, Z., Liu, P., Han, J., and Harding, I.C., Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies, Pharmacol. Rev., 2021, vol. 73, no. 3, pp 924–967.
Aird, W.C., Review article The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome, Blood, 2003, vol. 101, no. 10, pp. 3765–3777.
Prasad, M., Leon, M., Lerman, L.O., and Lerman, A., Viral endothelial dysfunction: A unifying mechanism for COVID-19, Mayo Clin. Proc., 2021, vol. 96, no. 12, pp. 3099–3108.
Jin, Y., Ji, W., Yang, H., Chen, S., Zhang, W., and Duan, G., Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches, Signal Transduction Targeted Ther., 2020, vol. 5, no. 1, p. 293.
Birnhuber, A., Fliesser, E., Gorkiewicz, G., Zacharias, M., Seeliger, B., David, S., Welte, T., Schmidt, J., Olschewski, H., Wygrecka, M., and Kwapiszewska, G., Between inflammation and thrombosis: endothelial cells in COVID-19, Eur. Respir. J., 2021, vol. 58, no. 3, p. 2100377.
Otifi, H.M. and Adiga, B.K., Endothelial dysfunction in COVID-19 infection, Am. J. Med. Sci., 2022, vol. 363, no. 4, pp. 285–291.
Panieri, E. and Santoro, M.M., ROS signaling and redox biology in endothelial cells, Cell. Mol. Life Sci., 2015, vol. 72, no. 17, pp. 3281–3303.
Aldosari, S., Awad, M., Harrington, E.O., Sellke, F.W., and Abid, M.R., Subcellular reactive oxygen species (ROS) in cardiovascular pathophysiology, Antioxidants, 2018, vol. 7, no. 1, p. 14.
Didion, S.P., Cellular and oxidative mechanisms associated with interleukin-6 signaling in the vasculature, Int. J. Mol. Sci., 2017, vol. 18, no. 12, p. 2563.
Volk, T., Hensel, M., Schuster, H., and Kox, W.J., Secretion of MCP-1 and IL-6 by cytokine stimulated production of reactive oxygen species in endothelial cells, Mol. Cell. Biochem., 2000, vol. 206, no. 1–2, pp. 105–112.
Ali, M.H., Schlidt, S.A., Chandel, N.S., Hynes, K.L., Schumacker, P.T., and Gewertz, B.L., Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction, Am. J. Physiol.: Lung Cell. Mol. Physiol., 1999, vol. 277, no. 5, pp. L1057–L1065.
Lee, Y.W., Lee, W.H., and Kim, P.H., Oxidative mechanisms of IL-4-induced IL-6 expression in vascular endothelium, Cytokine, 2010, vol. 49, no. 1, pp. 73–79.
Pearlstein, D.P., Ali, M.H., Mungai, P.T., Hynes, K.L., Gewertz, B.L., and Schumacker, P.T., Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia, Arterioscler., Thromb., Vasc. Biol., 2002, vol. 22, no. 4, pp. 566–573.
Zinovkin, R.A., Romaschenko, V.P., Galkin, I.I., Zakharova, V.V., Pletjushkina, O.Y., Chernyak, B.V., and Popova, E.N., Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium, Aging (Albany), 2014, vol. 6, no. 8. pp. 661–674.
Zakharova, V.V., Pletjushkina, O.Y., Galkin, I.I., Zinovkin, R.A., Chernyak, B.V., Krysko, D.V., Bachert, C., Krysko, O., Skulachev, V.P., and Popova, E.N., Low concentration of uncouplers of oxidative phosphorylation decreases the TNF-induced endothelial permeability and lethality in mice, Biochim. Biophys. Acta, Mol. Basis Dis., 2017, vol. 1863, no. 4, pp. 968–977.
Wassmann, S., Stumpf, M., Strehlow, K., Schmid, A., Schieffer, B., Böhm, M., and Nickenig, G., Interleukin-6 induces oxidative stress and endothehal dysfunction by overexpression of the angiotensin II type 1 receptor, Circ. Res., 2004, vol. 94, no. 4, pp. 534–541.
Chopra, A., Willmore, W.G., and Biggar, K.K., Protein quantification and visualization via ultraviolet-dependent labeling with 2,2,2-trichloroethanol, Sci. Rep., 2019, vol. 9, p. 13923.
Li, R., Ren, T., and Zeng, J., Mitochondrial coenzyme Q protects sepsis-induced acute lung injury by activating PI3K/Akt/GSK-3β/mTOR pathway in rats, BioMed Res. Int., 2019, vol. 2019, p. 5240898.
Zakharova, V.V., Pletjushkina, O.Y., Zinovkin, R.A., Popova, E.N., and Chernyak, B.V., Mitochondria-targeted antioxidants and uncouplers of oxidative phosphorylation in treatment of the systemic inflammatory response syndrome (SIRS), J. Cell. Physiol., 2017, vol 232, no. 5, pp. 904–912.
Rademann, P., Weidinger, A., Drechsler, S., et al., Mitochondria-targeted antioxidants SkQ1 and MitoTEMPO failed to exert a long-term beneficial effect in murine polymicrobial sepsis, Oxid. Med. Cell. Longevity, 2017, vol. 2017, p. 6412682.
Ding, J., Song, D., Ye, X., and Liu, S.F., A pivotal role of endothelial-specific NF-κB signaling in the pathogenesis of septic shock and septic vascular dysfunction, J. Immunol., 2009, vol. 183, no. 6, pp. 4031–4038.
Demyanenko, I.A., Popova, E.N., Zakharova, V.V., Ilyinskaya, O.P., Vasilieva, T.V., Romashchenko, V.P., Fedorov, A.V., Manskikh, V.N., Skulachev, M.V., Zinovkin, R.A., Pletjushkina, O.Y., Skulachev, V.P., and Chernyak, B.V., Mitochondria-targeted antioxidant SkQ1 improves impaired dermal wound healing in old mice, Aging (Albany), 2015, vol. 7, no. 7, pp. 475–485.
Galkin, I.I., Pletjushkina, O.Y., Zinovkin, R.A., Zakharova, V.V., Chernyak, B.V., and Popova, E.N., Mitochondria-targeted antioxidant SkQR1 reduces TNF-induced endothelial permeability in vitro, Biochemistry (Moscow), 2016, vol. 81, no. 10, pp. 1188–1197.
Galkin, I.I., Pletjushkina, O.Y., Zinovkin, R.A., Zakharova, V.V., Birjukov, I.S., Chernyak, B.V., Popova E.N. Mitochondria-targeted antioxidants prevent TNFα-induced endothelial cell damage, Biochemistry (Moscow), 2014, vol. 79, no. 2, pp. 124–130.
Chelombitko, M.A., Averina, O.A., Vasilyeva, T.V., Pletiushkina, O.Y., Popova, E.N., Fedorov, A.V., Chernyak, B.V., Shishkina, V.S., and Ilinskaya, O.P., Mitochondria-targeted antioxidant SkQ1 (10-(6′-plastoquinonyl)decyltriphenylphosphonium bromide) inhibits mast cell degranulation in vivo and in vitro, Biochemistry (Moscow), 2017, vol. 82, no. 12, pp. 1493–1503.
Chelombitko, M.A., Averina, O.A., Vasil’eva, T.V., Dvorianinova, E.E., Egorov, M.V., Pletjushkina, O.Y., Popova, E.N., Fedorov, A.V., Romashchenko, V.P., and Ilyinskaya, O.P., Comparison of the effects of mitochondria-targeted antioxidant 10-(6’-plastoquinonyl)decyltriphenylphosphonium bromide (SkQ1) and a fragment of its molecule dodecyltriphenylphosphonium on carrageenan-induced acute inflammation in mouse model of subcuteneo, Bull. Exp. Biol. Med., 2017, vol. 162, no. 6, pp. 730–733.
Funding
The research was funded by Russian Foundation for Basic Research, project number 20-04-60452.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
STUDY LIMITATIONS
The work was performed on the EA.hy926 cell line, which is a hybrid primary culture of human umbilical vein endothelial cells (HUVEC) and A549 human lung tissue adenocarcinoma.
Additional information
Translated by P. Kuchina
About this article
Cite this article
Chelombitko, M.A., Galkin, I.I., Pletjushkina, O.Y. et al. Effect of Antioxidants on the Production of MCP-1 Chemokine by EA.hy926 Cells in Response to IL-6. Moscow Univ. Biol.Sci. Bull. 77, 184–191 (2022). https://doi.org/10.3103/S0096392522030026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0096392522030026