Abstract
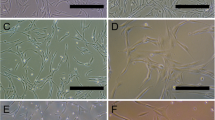
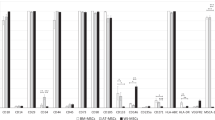
A promising direction in the development of new technologies for the treatment of the central nervous system diseases is the use of different types of stem cells, in particular, mesenchymal multipotent stromal cells (MMSCs) and neurogenic stem/progenitor cells (NSCs/NPCs). An alternative to direct cell transplantation may be the use of their conditioned media (CM) as a source of secretome and a key component of the mechanism of realization of their potential. Currently, in clinical trials using cell therapy in CNS pathology, the adipose tissue, bone marrow, umbilical cord and cord blood are most commonly used as a source for isolation of the MMSCs or mononuclear cells of the stromal-vascular fraction, and as a source of the NSCs/NPCs the lines of immortalized neurogenic cells isolated from structures of the brain or spinal cord of the human embryo are used. In experimental conditions, in particular in rodents, one of the most available sources of allogenic progenitor cells of the mesenchymal type is adipose tissue, and fetal brain is accessible source of neurogenic cells. The aim was to study the neuroregenerative effects of conditioned media from rat adipose tissue-derived fibroblast-like cells and fetal neurogenic cells in vitro. Methods. CM from 24 h cultures of rat adipose tissue-derived fibroblast-like cells (ad-FLCs) and fetal neurogenic cells (NCs, E14) were examined by electrophoresis in 10% polyacrylamide gel. On 5–7 day in 2D cultures of rat neural cells (E14) the “scratch assay” was performed and nutrient medium DMEM with 10% fetal calf serum (standard culture conditions, control) or 0.10 mg/mL (by amount of protein) ad-FLCs CM or NCs CM were added. Microscopic and morphometric studies were performed during 4-day cultivation. Results. After mechanical transection in the culture of neural cells under standard conditions from the 1 to the 3 day there were processes of endogenous regeneration, which decreased to the 4 day. The addition of ad-FLCs CM or NCs CM contributed to a significant increase in the degree and duration of endogenous regeneration processes in neural cell culture. Exposure to ad-FLCs CM increased to the 4 day in the scratch area the number of migrated cells (7 times) and the density of cell processes (12.5 times); exposure to NCs CM increased the number of migrated cells (3.5 times), the distance of cell migration (1.4 times), the density of cell processes (13 times). The length of the overgrown section of the scratch area increased after exposure to ad-FLCs CM in 1.7 times, NCs CM—3 times, reaching respectively 23.7 and 43.5% of the total length of the transection zone. 10 protein fractions were detected in the ad-FLCs CM: predominant 12, 15, 23, 30, 80 kDa and minor—28, 35, 55, 65, 75 kDa; in NCs CM—9 fractions: prevailing 15, 23, 30, 35 kDa and minor—37, 40, 46, 67, 80 kDa. Conclusions. CM from 24 h cultures of rat ad-FLCs or NCs (E14) stimulate endogenous regeneration processes in rat brain cell culture with mechanical monolayer transection. The affecting factors of neuroregenerative “bystander” effects of rat ad-FLCs or NCs are secreted biologically active proteins—components of the CM predominant and minor protein fractions.




Similar content being viewed by others
REFERENCES
Bao, X., Feng, M., Wei, J. et al., Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes angiogenesis and neurogenesis after cerebral ischemia in rats, Eur. J. Neurosci., 2011, vol. 34, pp. 87–98. https://doi.org/10.1111/j.1460-9568.2011.07733.x
Beretta, S., Cunningham, K.M., Haus, D.L., et al., Effects of human ES-derived neural stem cell transplantation and kindling in a rat model of traumatic brain injury, Cell Transplant., 2017, vol. 26, pp. 1247–1261. https://doi.org/10.1177/0963689717714107
Cunningham, C.J., Enrich, M.V., Pickford, M.M. et al., The therapeutic potential of the stem cell secretome for spinal cord repair: a systematic review and meta-analysis, OBM Neurobiol., 2020, vol. 4, no. 4. https://doi.org/10.21926/obm.neurobiol.2004080
Dobrowolski, S. and Lepski, G., Stem cells in traumatic brain injury, Am. J. Neurosci., 2013, vol. 4, pp. 13–24. https://doi.org/10.3844/ajnsp.2013.13.24
Gao, J., Grill, R.J., Dunn, T.J., et al., Human neural stem cell transplantation-mediated alteration of microglial/macrophage phenotypes after traumatic brain injury, Cell Transplant., 2016, vol. 25, pp. 1863–1877. https://doi.org/10.3727/096368916X691150
Gomazkov, O.A., Neurogenesis as an adaptive function of the brain, Biol. Bull. Rev., 2014, vol. 4, pp. 86–100.
Haus, D.L., López-Velázquez, L., Gold, E.M., et al., Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury, Exp. Neurol., 2016, vol. 281, pp. 1–16. https://doi.org/10.1016/j.expneurol.2016.04.008
Kocan, B., Maziarz, A., Tabarkiewicz, J., et al., Trophic activity and phenotype of adipose tissue-derived mesenchymal stem cells as a background of their regenerative potential, Stem Cells Int., 2017, vol. 2017, art. ID 1653254. https://doi.org/10.1155/2017/1653254
Kopach, O. and Pivneva, T., Cell-based therapies for neural replacement strategies in stroke-related neurodegeneration: neurophysiological insights into stem progenitor cell neurogenesis within a host environment, Neural Regener. Res., 2018, vol. 13, pp. 1350–1351. https://doi.org/10.4103/1673-5374.235224
Lee, M.C., Jin, C.Y., Kim, H.S., et al., Stem cell dynamics in an experimental model of stroke, Chonnam Med. J., 2011, vol. 47, no. 2, pp. 90–98. https://doi.org/10.4068/cmj.2011.47.2.90
Ludwig, P.E., Thankam, F.G., Patil, A.A., et al., Brain injury and neural stem cells, Neural Regener. Res., 2018, vol. 13, no. 1, pp. 7–18. https://doi.org/10.4103/1673-5374.224361
Qu, X. and Sheng, H., Stem cell therapy for traumatic brain injury: a progress update, Ann. Neurol. Surg., 2018, vol. 2, art. ID 1008.
Semenova, V., Lisianyi, N., Stayno, L., et al., Proliferative and differentiated potential of mesenchymal stem cells from adipose tissue under cultivation conditions, Ukr. Neurosurg. J., 2014, vol. 3, pp. 24–29 https://doi.org/10.25305/unj.47487
Shi, W., Huang, C.J., Xu, X.D., et al., Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury, Acta Biomater., 2016, vol. 45, pp. 247–261. https://doi.org/10.1016/j.actbio.2016.09.001
Walker, P.A., Letourneau, P.A., Bedi, S., et al., Progenitor cells as remote “bioreactors”: Neuroprotection via modulation of the systemic inflammatory response, World J. Stem Cells, 2011, vol. 3, pp. 9–18. https://doi.org/10.4252/wjsc.v3.i2.9
Webb, R.L., Kaiser, E.E., Spellicy, S., et al., Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model, Transl. Stroke Res., 2018, vol. 9, pp. 530–539. https://doi.org/10.1007/s12975-017-0599-2
Wei, L., Fraser, J.L., Lu, Z.Y., et al., Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats, Neurobiol. Dis., 2012, vol. 46, no. 3, pp. 635–645. https://doi.org/10.1016/j.nbd.2012.03.002
Willis, C.M., Nicaise, A.M., Hamel, R., et al., Harnessing the neural stem cell secretome for regenerative neuroimmunology, Front. Cell Neurosci., 2020, vol. 14, art. ID 590960. https://doi.org/10.3389/fncel.2020.590960
Xu, Ch., Diao, Y.F., Wang, J., et al., Intravenously infusing the secretome of adipose-derived mesenchymal stem cells ameliorates neuroinflammation and neurological functioning after traumatic brain injury, Stem Cells Dev., 2020, vol. 29, pp. 222–234. https://doi.org/10.1089/scd.2019.0173
Yang, H., Wang, C., Chen, H., et al., Neural stem cell-conditioned medium ameliorated cerebral ischemia-reperfusion injury in rats, stem cells international, 2018, vol. 2018, art. ID 4659159. https://doi.org/10.1155/2018/4659159
Zhong, D., Cao, Y., Li, C.J., et al., Neural stem cell-derived exosomes facilitate spinal cordfunctional recovery after injury by promoting angiogenesis, Exp. Biol. Med., 2020, vol. 245, no. 1, pp. 54–65. https://doi.org/10.1177/1535370219895491
Funding
The work is performed within the framework of research project funding, state registration no. 0119U000114.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. The study was approved by the Commission on Ethics and Bioethics of the State Institution “Romodanov Neurosurgery Institute, National Academy of Medical Sciences of Ukraine” (protocol no. 26 of May 11, 2018).
About this article
Cite this article
Pedachenko, E.G., Liubich, L.D., Staino, L.P. et al. Neuroregenerative “Bystander”-Effects of Conditioned Media from Adipose Tissue-Derived Fibroblast-Like Cells in Vitro. Cytol. Genet. 56, 139–147 (2022). https://doi.org/10.3103/S0095452722020098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722020098