Abstract
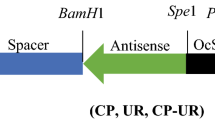
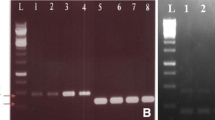
Potato virus Y (PVY) is among the most destructive potato viruses and a solemn threat to efficient seed production worldwide. Almost 70% potato yield losses are due to early PVY infection. Coat protein (CP) gene has a significant function in host adaptation in most of the plant viruses. CP has become more frequent and extensively studied in PVY and other potyviruses to generate resistance against the potyviruses in different crops. In this study, we also targeted the CP gene and evaluate the in vitro potential of Iberis gibraltarica total protein extract against the CP gene expression. Total protein of Iberis gibraltarica, was isolated and quantified. The full length CP gene was amplified from PVY infected potato plant leaves and cloned into mammalian expression vector. It was later transfected into mammalian cell line to obtain the transient expression and mRNA expression of the CP gene against the total protein of Iberis gibraltarica was analyzed through the real-time PCR. Results show that total protein of Iberis gibraltarica down regulates the mRNA expression more than 90% in vitro studies.




Similar content being viewed by others
REFERENCES
Abbas, A. and Amrao, L., Potato virus Y: an evolving pathogen of potato worldwide, Pak J. Phytopathol., 2017, vol. 29, no. 1, pp. 187–191.
Ali, M., Rafique, F., Ali, Q., and Malik, A., Genetic modification for salt and drought tolerance in plants through SODERF3, Biol. Clin. Sci. Res. J., 2020a, vol. 2020. e022.
Ali, Q., Khalil, R., Nadeem, M., Azhar, M., Hafeez, M., and Malik, A., Antibacterial, antioxidant activities and association among plant growth related traits of Lepidium draba, Biol. Clin. Sci. Res. J., 2020b, vol. 2020. e011.
Asif, S., Ali, Q., and Malik, A., Evaluation of salt and heavy metal stress for seedling traits in wheat, Biol. Clin. Sci. Res. J., 2020, vol. 2020. e005.
Azizgolshani, O., Garmann, R.F., Cadena-Nava, R., Knobler, C.M., and Gelbart, W.M., Reconstituted plant viral capsids can release genes to mammalian cells, Virology, 2013, vol. 441, no. 1, pp. 12–17.
Bashir, A., Ali, Q., Rashid, M., and Malik, A., CSRISPR-Cas9 in genome editing: a nature gifted molecular tool, Biol. Clin. Sci. Res. J., 2020, vol. 2020. e018.
Bilal, M., Tabassum, B., Farooq, A.M., Tariq, M., Nasir, I.A., and Hussnain, T., In-vitro analysis of Chenopodium murale extract for resistance against potato virus Y, Pure Appl. Biol., 2019, vol. 8, no. 2, pp. 1172–1181.
Bol, J.F., Role of capsid proteins, in Plant Virology Protocols, Springer, 2008, pp. 21–31.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, nos. 1–2, pp. 248–254.
Butt, S.J., Varis, S., Nasir, I.A., Sheraz, S., and Shahid, A., Micro propagation in advanced vegetable production: a review, Adv. Life Sci., 2015, vol. 2, no. 2, pp. 48–57.
Chaudhary, K., CRISPR/Cas13a targeting of RNA virus in plants, Plant Cell Rep., 2018, vol. 37, no. 12, pp. 1707–1712.
Choudhary, N., Kapoor, H.C., and Lodha, M.L., Cloning and expression of antiviral/ribosome-inactivating protein from Bougainvillea xbuttiana, J. Biosci., 2008, vol. 33, no. 1, pp. 91–101.
Chung, B.Y.-W., Miller, W.A., Atkins, J.F., and Firth, A.E., An overlapping essential gene in the Potyviridae, Proc. Natl. Acad. Sci. U. S. A., 2008, vol. 105, no. 15, pp. 5897–5902.
Danish, P., Ali, Q., Hafeez, M., and Malik, A., Antifungal and antibacterial activity of aloe vera plant extract, Biol. Clin. Sci. Res. J., 2020, vol. 2020. e003.
Davie, K., Holmes, R., Pickup, J., and Lacomme, C., Dynamics of PVY strains in field grown potato: Impact of strain competition and ability to overcome host resistance mechanisms, Virus Res., 2017, vol. 241, pp. 95–104.
Deja-Sikora, E., Mercy, L., Baum, C., and Hrynkiewicz, K., The contribution of endomycorrhiza to the performance of potato virus Y-Infected solanaceous plants: disease alleviation or exacerbation?, Front. Microbiol., 2019, vol. 10, p. 516.
Devaux, A., Kromann, P., and Ortiz, O., Potatoes for sustainable global food security, Potato Res., 2014, vol. 57, nos. 3–4, pp. 185–199.
Elbeshehy, E.K., Inhibitor activity of different medicinal plants extracts from Thuja orientalis, Nigella sativa L., Azadirachta indica and Bougainvillea spectabilis against Zucchini yellow mosaic virus (ZYMV) infecting Citrullus lanatus, Biotechnol. Biotechnol. Equip., 2017, vol. 31, no. 2, pp. 270–279.
Elbeshehy, E.K., Metwali, E.M., and Almaghrabi, O.A., Antiviral activity of Thuja orientalis extracts against watermelon mosaic virus (WMV) on Citrullus lanatus, Saudi J. Biol. Sci., 2015, vol. 22, no. 2, pp. 211–219.
Gargouri-Bouzid, R., Jaoua, L., Mansour, R.B., Yemna, H., Malika, A., and Radhouane, E., PVY resistant transgenic potato plants (cv Claustar) expressing the viral coat protein, J. Plant Biotechnol., 2005, vol. 7, no. 3, pp. 1–6.
Hameed, A., Iqbal, Z., and Shaheen Asad, S.M., Detection of multiple potato viruses in the field suggests synergistic interactions among potato viruses in Pakistan, Plant Pathol. J., 2014, vol. 30, no. 4, p. 407.
HorvAth, J., New artificial hosts and non-hosts of plant viruses and their role in the identification and separation of viruses XVIII. Concluding remarks, Acta Phytopathol., 1983, vol. 18, nos. 1–3, pp. 121—161.
Hull, R., Plant Virology, Academic, 2013.
Incarbone, M. and Dunoyer, P., RNA silencing and its suppression: novel insights from in planta analyses, Trends Plant Sci., 2013, vol. 18, no. 7, pp. 382–392.
Iqbal, M.S., Jabbar, B., Sharif, M.N., Ali, Q., Husnain, T., and Nasir, I.A., In silico MCMV silencing concludes potential host-derived miRNAs in maize, Front. Plant Sci., 2017, vol. 8, p. 372.
Janzac, B., Tribodet, M., Lacroix, C., Moury, B., Verrier, J., and Jacquot, E., Evolutionary pathways to break down the resistance of allelic versions of the PVY resistance gene va, Plant Dis., 2014, vol. 98, no. 11, pp. 1521–1529.
Karasev, A.V. and Gray, S.M., Continuous and emerging challenges of Potato virus Y in potato, Ann. Rev. Phytopathol., 2013, vol. 51, pp. 571–586.
Kenesi, E., Carbonell, A., Lózsa, R., Vértessy, B., and Lakatos, L., A viral suppressor of RNA silencing inhibits ARGONAUTE 1 function by precluding target RNA binding to pre-assembled RISC, Nucleic Acids Res., 2017, vol. 45, no. 13, pp. 7736–7750.
Khalil, R., Ali, Q., Hafeez, M., and Malik, A., Phenolic acid profiling by RP-HPLC: evaluation of antibacterial and anticancer activities of Conocarpus erectus plant extracts, Biol. Clin. Sci. Res. J., 2020a, vol. 2020. e010.
Khalil, R., Ali, Q., Hafeez, M., and Malik, A., Phytochemical activities of Conocarpus erectus: an overview, Biol. Clin. Sci. Res. J., 2020b, vol. 2020. e008.
Li, L., Wang, L., Xiao, R., Zhu, G., Li, Y., Liu, C., Yang, R., Tang, Z., Li, J., and Huang, W., The invasion of tobacco mosaic virus RNA induces endoplasmic reticulum stress-related autophagy in HeLa cells, Biosci. Rep., 2012, vol. 32, no. 2, pp. 171–184.
Lockney, D.M., Guenther, R.N., Loo, L., Overton, W., Antonelli, R., Clark, J., Hu, M., Luft, C., Lommel, S.A., and Franzen, S., The red clover necrotic mosaic virus capsid as a multifunctional cell targeting plant viral nanoparticle, Bioconjugate Chem., 2011, vol. 22, no. 1, pp. 67–73.
Loebenstein, G. and Gaba, V., Viruses of potato, Adv. Virus Res., Elsevier, 2012, vol. 84, pp. 209–246.
Mosmann, T., Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, vol. 65, nos. 1–2, pp. 55–63.
Moury, B., Simon, V., Faure, C., Svanella-Dumas, L., Marais, A., and Candresse, T., Host groups of Potato virus Y: vanishing barriers, in Potato Virus Y: Biodiversity, Pathogenicity, Epidemiology and Management, Springer, 2017, pp. 243–261.
Muhammad, S., Shahbaz, M., Iqbal, M., Wahla, A.S., Ali, Q., Shahid, M.T.H., and Tariq, M.S., Prevalence of different foliar and tuber diseases on different varieties of potato, Adv. Life Sci., 2013, vol. 1, no. 1.
Mushtaq, U., Mushtaq, S., Afzal, M., Ali, Q., and Malik, A., Role of modern technology for treatment of HCV, Biol. Clin. Sci. Res. J., 2020, vol. 2020. e001.
Nadeem, A., Khan, M., Safdar, A., Khan, N., Rana, B., and Sandhu, A., Epidemiological studies and management of potato germplasm against PVX and PVY, Pakistan J. Phytopathol., 2011, vol. 23, no. 2, pp. 159–165.
Petrov, N.M., Antiviral activity of plant extract from Tanacetum vulgare against cucumber mosaic virus and potato virus Y, J. Biosci. Biotechnol., 2016, vol. 5, no. 2, pp. 189–194.
Petrov, N., Stoyanova, M., Andonova, R., and Teneva, A., Induction of resistance to potato virus Y strain NTN in potato plants through RNAi, Biotechnol. Biotechnol. Equip., 2015, vol. 29, no. 1, pp. 21–26.
Pooggin, M.M., RNAi-mediated resistance to viruses: a critical assessment of methodologies, Curr. Opin. Virol., 2017, vol. 26, pp. 28–35.
Quenouille, J., Vassilakos, N., and Moury, B., Potato virus Y: a major crop pathogen that has provided major insights into the evolution of viral pathogenicity, Mol. Plant Pathol., 2013, vol. 14, no. 5, pp. 439–452.
Rehman, S., Ashfaq, U.A., Riaz, S., Javed, T., and Riazuddin, S., Antiviral activity of Acacia nilotica against hepatitis C virus in liver infected cells, Virol. J., 2011, vol. 8, no. 1, p. 220.
Rio, D.C., Ares, M., Hannon, G.J., and Nilsen, T.W., Purification of RNA using TRIzol (TRI reagent), Cold Spring Harbor Protocols, 2010, vol. 6. pdb.prot5439.
Shabana, Y.M., Abdalla, M.E., Shahin, A.A., El-Sawy, M.M., Draz, I.S., and Youssif, A.W., Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina, Egypt. J. Basic Appl. Sci., 2017, vol. 4, no. 1, pp. 67–73.
Tabassum, B., Nasir, I.A., and Husnain, T., Potato virus Y mRNA expression knockdown mediated by siRNAs in cultured mammalian cell line, Virol. Sin., 2011, vol. 26, no. 2, pp. 105–113.
Tahir, T., Ali, Q., Rashid, M., and Malik, A., The journey of CRISPR-Cas9 from bacterial defense mechanism to a gene editing tool in both animals and plants, Biol. Clin. Sci. Res. J., 2020. e017.
Takakura, Y., Udagawa, H., Shinjo, A., and Koga, K., Mutation of a Nicotiana tabacum L. eukaryotic translation-initiation factor gene reduces susceptibility to a resistance-breaking strain of potato virus Y, Mol. Plant Pathol., 2018, vol. 19, no. 9, pp. 2124–2133.
Wei, T., Zhang, C., Hong, J., Xiong, R., Kasschau, K.D., Zhou, X., Carrington, J.C., and Wang, A., Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO, PLoS Pathog., 2010, vol. 6, no. 6. e1000962.
Whitham, S.A., Anderberg, R.J., Chisholm, S.T., and Carrington, J.C., Arabidopsis RTM2 gene is necessary for specific restriction of tobacco etch virus and encodes an unusual small heat shock-like protein, Plant Cell, 2000, vol. 12, no. 4, pp. 569–582.
Wylie, S.J., Adams, M., Chalam, C., Kreuze, J., Lopez-Moya, J.J., Ohshima, K., Praveen, S., Rabenstein, F., Stenger, D., and Wang, A., ICTV virus taxonomy profile: Potyviridae, J. Gen. Virol., 2017, vol. 98, no. 3, p. 352.
Yamaji, Y., Maejima, K., Komatsu, K., Shiraishi, T., Okano, Y., Himeno, M., Sugawara, K., Neriya, Y., Minato, N., and Miura, C., Lectin-mediated resistance impairs plant virus infection at the cellular level, Plant Cell, 2012, vol. 24, no. 2, pp. 778–793.
Yang, L., Wang, X., Deng, W., Mo, W., Gao, J., Liu, Q., Zhang, C., Wang, Q., Lin, C., and Zuo, Z., Using HEK293T expression system to study photoactive plant cryptochromes, Front. Plant Sci., 2016, vol. 7, p. 940.
Yaqoob, S., Fatima, N., Khan, S., Ali, Q., Hafeez, M., and Malik, A., Begomoviruses and betasatellites associated with CLCuD, Biol. Clin. Sci. Res. J., 2020, vol. 2020. e002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
About this article
Cite this article
Bilal, M., Tabassum, B., Ali, Q. et al. Down Regulation of Potato Virus Y (PVY) Coat Protein (CP) Expression by Iberis gibraltarica Protein Extract. Cytol. Genet. 55, 80–86 (2021). https://doi.org/10.3103/S0095452721010102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721010102