Abstract
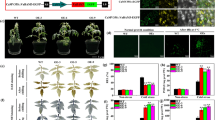
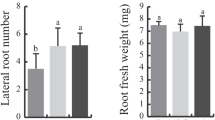
SnRK1 protein kinases are integral components of cell signaling involved in stress response, regulation of energy metabolism, seed germination and maturation, autophagy, and other processes. Nevertheless, many functions of these protein kinases remain unknown. In order to study the intracellular localization of KIN10 (catalytic subunit of the SnRK1 protein kinase complexes), protoplasts of the wild A. thaliana ecotype (Col-0) were transformed with a construct that included the KIN10-RFP fusion gene. It was established that the chimeric KIN10-RFP protein was diffusely distributed in the cytoplasm; however, it was mainly localized on the cell periphery, in the region adjacent to the plasma membrane. The diffuse distribution of KIN10 and γ-tubulin in the cytoplasm of A. thaliana root cells was demonstrated by immunofluorescence microscopy. The patterns of γ-tubulin intracellular localization in the root cells of the kin10 and kin11 knockout mutants and wild type A. thaliana were shown to be different. The intensity of γ-tubulin fluorescence in both mutants was lower than in the wild type-plants: this may be indicative of certain impairments in the formation of γ-tubulin complexes in the mutants. A lower intensity of γ-tubulin fluorescence was also recorded in cells of all lines cultivated under the conditions of energy shortage. In particular, the lowest level of fluorescence was recorded in the kin10 and kin11 mutants exposed to this type of stress, and this may be indicative of the synergistic influence of simultaneous dysfunction of one of these genes and energy deficiency on the formation of γ-tubulin complexes (γTuSC and γTuRC). The results obtained demonstrate the possible involvement of SnRK1 (KIN10 and KIN11) in the physiological response of plants to energy stress. With the data on the possible phosphorylation of the Ser131 residue in plant γ-tubulin by KIN10 taken into account, it can be assumed that SnRK1 protein kinases are involved in the regulation of microtubule polymerization in plants.




Similar content being viewed by others
REFERENCES
Polge, C. and Thomas, M., SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control?, Trends Plant Sci., 2007, vol. 21, no. 1, pp. 20–28. https://doi.org/10.1016/j.tplants.2006.11.005
Lumbreras, V., Alba, M.M., Kleinow, T., Koncz, C., and Pages, M., Domain fusion between SNF1-related kinase subunits during plant evolution, EMBO Rep., 2001, vol. 2, no. 1, pp. 55–60. https://doi.org/10.1093/emboreports/kve001
Cho, H.Y., Wen, T.N., Wang, Y.T., and Shih, M.C., Quantitative phosphoproteomics of protein kinase SnRK1 regulated protein phosphorylation in Arabidopsis under submergence, J. Exp. Bot., 2016, vol. 67, no. 9, pp. 2745–2760. https://doi.org/10.1093/jxb/erw107
Crozet, P., Margalha, L., Confraria, A., Rodrigues, A., Martinho, C., Adamo, M., Elias, C.A., and Baena-González, E., Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases, Front. Plant Sci., 2014, no. 5. https://doi.org/10.3389/fpls.2014.00190
Hardie, D.G., AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy, Nat. Rev. Mol. Cell Biol., 2007, vol. 8, no. 10, pp. 774–785. https://doi.org/10.1038/nrm2249
Broeckx, T., Hulsmans, S., and Rolland, F., The plant energy sensor: evolutionary conservation and divergence of SnRK1 structure, regulation, and function, J. Exp Bot., 2016, vol. 67, no. 22, pp. 6215–6252. https://doi.org/10.1093/jxb/erw416
Baena-González, E., Rolland, F., Thevelein, J.M., and Sheen, J., A central integrator of transcription networks in plant stress and energy signalling, Nature, 2007, vol. 448, no. 7156, pp. 938–942. https://doi.org/10.1038/nature06069
Zhang, Y., Primavesi, L.F., Jhurreea, D., Andralojc, P.J., Mitchell, R.A., Powers, S.J., Schluepmann, H., Delatte, T., Wingler, A., and Paul, M.J., Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate, Plant Physiol., 2009, vol. 149, no. 4, pp. 1860–1871. https://doi.org/10.1104/pp.108.133934
Nagele, T. and Weckwerth, W., Mathematical modeling reveals that metabolic feedback regulation of SnRK1 and hexokinase is sufficient to control sugar homeostasis from energy depletion to full recovery, Front. Plant Sci., 2014, vol. 5, no. 365, https://doi.org/10.3389/fpls.2014.00365
Nunes, C., O’Hara, L.E., Primavesi, L.F., Delatte, T.L., Schluepmann, H., Somsen, G.W., Silva, A.B., Fevereiro, P.S., Wingler, A., and Paul, M.J., The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation, Plant Physiol., 2013, vol. 162, no. 3, pp. 1720–1732. https://doi.org/10.1104/pp.113.220657
Mair, A., Pedrotti, L., Wurzinger, B., Anrather, D., Simeunovic, A., Weiste, C., Valerio, C., Dietrich, K., Kirchler, T., Nägele, T., Carbajosa, J.V., Hanson, J., Baena-González, E., Chaban, C., Weckwerth, W., Dröge-Laser, W., and Teige, M., SnRK1-triggered switch of bZIP63 dimerization mediates the lowenergy response in plants, eLife, 2015. https://doi.org/10.7554/eLife.05828
Nukarinen, E., Nägele, T., Pedrotti, L., Wurzinger, B., Mair, A., Landgraf, R., Börnke, F., Hanson, J., Teige, M., Baena-González, E., Dröge-Laser, W., and Weckwerth, W., Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation, Sci. Rep., 2016, vol. 6. https://doi.org/10.1038/13.srep31697
Soto-Burgos, J. and Bassham, D.C., SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana, PLoS One, 2017, vol. 12, no. 8, e0182591. https://doi.org/10.1371/journal.pone.0182591
Krasnoperova, E.E., Buy, D.D., Goriunova, I.I., Isayenkov, S.V., Karpov, P.A., Blume, Ya.B., and Yemets, A.I., Potential role of protein kinases SNRK1 in regulation of cell division of Arabidopsis thaliana, Cytol. Genet., 2019. vol. 53, no. 3 (in press).
Krasnoperova, E.E., Isayenkov, S.V., Karpov, P.A., and Yemets, A.I., The cladistic analysis and characteristic of an expression of serine/threonine protein kinase KIN10 in different organs of Arabidopsis thaliana, Rep. Natl. Acad. Sci. Ukraine, 2016, no. 1, pp. 81–91. https://doi.org/10.15407/dopovidi2016.01.081
Karpov, P.A., Rayevsky, A.V., Krasnoperova, E.E., Isayenkov, S.V., Yemets, A.I., and Blume, Ya.B., Protein kinase KIN10 from Arabidopsis thaliana as a potential regulator of primary microtubule nucleation centers in plants, Cytol. Genet., 2017, vol. 51, no. 6, pp. 415–421. https://doi.org/10.3103/S0095452717060056
Mohannath, G., Jackel, J.N., Lee, Y.H., Buchmann, R.C., Wang, H., Patil, V., Adams, A.K., and Bisaro, D.M., A complex containing SNF1-related kinase (SnRK1) and adenosine kinase in Arabidopsis, PLoS One, 2014, vol. 149, no. 4, e87592. https://doi.org/10.1371/journal.pone.0087592
Jossier, M., Bouly, J.P., Meimoun, P., Arjmand, A., Lessard, P., Hawley, S., Grahame, HardieD., and Thomas, M., SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana, Plant J., 2009, vol. 59, no. 2, pp. 316–328. https://doi.org/10.1111/j.1365-313X.2009.03871.x
Yoo, S.-D., Cho, Y.-H., and Sheen, J., Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis, Nat. Protoc., 2007, vol. 2, no. 7, pp. 1565–1572. https://doi.org/10.1038/nprot.2007.199
Krasnoperova, E.E., Novozhylov, D.O., Blume, Y.B., and Isayenkov, S.V., Creating of the plasmid construction protein kinase AtKIN10, conjoint with RFP to study the cellular localization of this enzyme, Proc. Uman NUH, 2014, vol. 84, no. 1, pp. 95–100. http://nbuv.gov.ua/UJRN/zhpumus_2014_84_15.
Isayenkov, S.V., Isner, J.C., and Maathuis, F.J., Rice two-pore K+ channels are expressed in different types of vacuoles, Plant Cell, 2011, vol. 23, no. 2, pp. 756–768. https://doi.org/10.1105/tpc.110.081463
Szechyńska-Hebda, M., Wedzony, M., Dubas, E., Kieft, H., and van Lammeren, A., Visualisation of microtubules and actin filaments in fixed BY-2 suspension cells using an optimised whole mount immunolabelling protocol, Plant Cell Rep., 2006, vol. 25, no. 8, pp. 758–766. https://doi.org/10.1007/s00299-005-0089-y
Sheen, J., Master regulators in plant glucose signaling network, J. Plant Biol., 2014, vol. 57, no. 2, pp. 67–79. https://doi.org/10.1007/s12374-014-0902-7
Fragoso, S., Espíndola, L., Páez-Valencia, J., Gamboa, A., Camacho, Y., Martínez-Barajas, E., and Coello, P., SnRK1 isoforms AKIN10 and AKIN11 are differentially regulated in Arabidopsis plants under phosphate starvation, Plant Physiol., 2009, vol. 149, no. 4, pp. 1906–1916. https://doi.org/10.1104/pp.108.133298
Hulsmans, S., Rodriguez, M., De Coninck, B., and Rolland, F., The SnRK1 energy sensor in plant biotic interactions, Trends Plant Sci., 2016, vol. 21, no. 8, pp. 648–661. https://doi.org/10.1016/j.tplants.2016.04.008
Eklund, G., Lang, S., Glindre, J., Ehlén, A., and Alvarado-Kristensson, M., The nuclear localization of -tubulin is regulated by SadB-mediated phosphorylation, J. Biol. Chem., 2014, vol. 289, no. 31, pp. 21360–21373. https://doi.org/10.1074/jbc.M114.562389
Sample, V., Ramamurthy, S., Gorshkov, K., Ronnett, G.V., and Zhang, J., Polarized activities of AMPK and BRSK in primary hippocampal neurons, Mol. Biol. Cell, 2015, vol. 26, no. 10, pp. 1935–1946. https://doi.org/10.1091/mbc.E14-02-0764
Alvarado-Kristensson, M., Rodríguez, M.J., Silio, V., Valpuesta, J.M., and Carrera, A.C., SADB phosphorylation of γ-tubulin regulates centrosome duplication, Nat. Cell Biol., 2009, vol. 11, pp. 1081–1092. https://doi.org/10.1038/ncb1921
Dhumale, P., Menon, S., Chiang, J., and Puschel, A.W., The loss of the kinases SadA and SadB results in early neuronal apoptosis and a reduced number of progenitors, PLoS One, 2018, vol. 13, no. 4. e0196698. https://doi.org/10.1371/journal.pone.0196698
Eklund, G., Lang, S., Glindre, J., Ehlen, A., and Alvarado-Kristensson, M., The nuclear localization of γ-tubulin is regulated by SadB-mediated phosphorylation, J. Biol. Chem., 2014, vol. 289, no. 31, pp. 21360–21373. https://doi.org/10.1074/jbc.M114.562389
Kollman, J.M., Merdes, A., Mourey, L., and Agard, D.A., Microtubule nucleation by γ-tubulin complexes, Nat. Rev. Mol. Cell Biol., 2011, vol. 12, pp. 709–721. https://doi.org/10.1038/nrm3209
Yemets, A., Stelmakh, O., and Blume, Y.B., Effects of the herbicide isopropyl-N-phenyl carbamate on microtubules and MTOCs in lines of Nicotiana sylvestris resistant and sensitive to its action, Cell Biol Int., 2008, vol. 32, no. 6, pp. 623–629. https://doi.org/10.1016/j.cellbi.2008.01.012
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by S. Semenova
About this article
Cite this article
Krasnoperova, E.E., Goriunova, I.I., Isayenkov, S.V. et al. Potential Involvement of KIN10 and KIN11 Catalytic Subunits of the SnRK1 Protein Kinase Complexes in the Regulation of Arabidopsis γ-Tubulin. Cytol. Genet. 53, 349–356 (2019). https://doi.org/10.3103/S0095452719050104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452719050104