Abstract
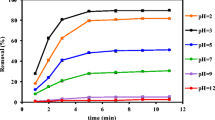
The boron-doped diamond electrode has been widely applied in electrochemical process for wastewater treatment based on the generation of hydroxyl radicals (•OH). This study investigated the formation efficiency of 2-HTA through the interaction between •OH radical and terephthalic acid (TA) to monitor the •OH formation. Results show that the 2-HTA formation efficiency did not depend on the initial concentration of scavenger (here, TA) in the investigated range of 0.1 to 1 mM TA. Meanwhile, other factors, such as current density, supporting electrolyte, sodium hydroxide have a significant influence. The optimal condition was established: concentration of electrolyte (0.05 M Na2SO4); initial concentration of TA (0.1 mM); concentration of NaOH (1 mM); current density (j = 20 mA cm–2). The selectivity of TA was evaluated by Faradaic efficiency (η) and hydroxylation yield (\({{{{\gamma }}}_{{{\text{TAOH}}}}}\)), indicating that Faradaic efficiency (η) for 2-HTA formation was in the range of 10–5–10–7, while the hydroxylation yield of •OH (\({{{{\gamma }}}_{{{\text{TAOH}}}}}\)) into 2-HTA was 0.03–0.12.










Similar content being viewed by others
REFERENCES
Sedlak, D.L. and Andren, A.W., Environ. Sci. Technol., 1991, vol. 25, no. 4, p. 777.
Hoang, N.T., Nguyen, X.C., Le, P.-C., Juzsakova, T., Chang, S.W., and Nguyen, D.D., J. Environ. Chem. Eng., 2021, vol. 9, no. 3, 105205. https://doi.org/10.1016/j.jece.2021.105205
Hoang, N.T. and Holze, R., Russ. J. Electrochem., 2020, vol. 56, no. 6, p. 492–505. https://doi.org/10.1134/S1023193520060087
Iniesta, J., Electrochim. Acta, 2001, vol. 46, no. 23, p. 3573. https://doi.org/10.1016/S0013-4686(01)00630-2
Sopchak, D., Miller, B., Avyigal, Y., and Kalish, R., J. Electroanal. Chem., 2002, vols. 538–539, p. 39. https://doi.org/10.1016/S0022-0728(02)01045-8
Hoang, N.T. and Holze, R., J. Solid State Electrochem., 2021, vol. 25, no. 1, p. 73. https://doi.org/10.1007/s10008-020-04581-7
Cañizares, P., Sáez, C., Lobato, J., and Rodrigo, M.A., Electrochim. Acta, 2004, vol. 49, no. 26, p. 4641. https://doi.org/10.1016/j.electacta.2004.05.019
Marselli, D., Garcia-Gomez, J., Michaud, P.-A., Rodrigo, M.A., and Comninellis, C., J. Electrochem. Soc., 2003, vol. 150, no. 3, p. D79. https://doi.org/10.1149/1.1553790
Gonzalez, D.H., Kuang, X.M., Scott, J.A., Rocha, G.O., and Paulson, S.E., Anal. Lett., 2018, vol. 51, no. 15, p. 2488, Oct. 2018. https://doi.org/10.1080/00032719.2018.1431246
Faust, B.C. and Allen, J.M., Environ. Sci. Technol., 1993, vol. 27, no. 6, p. 1221. https://doi.org/10.1021/es00043a024
Sato, M., Ohgiyama, T., and Clements, J.S., Proc. 1994 IEEE Industry Applications Society Annual Meeting, 1994, p. 1455. https://doi.org/10.1109/IAS.1994.377617
Ono, R. and Oda, T., J. Electrostat., 2002, vol. 55, nos. 3–4, p. 333. https://doi.org/10.1016/S0304-3886(01)00215-7
Černigoj, U., Štangar, U.L., Trebše, P., and Sarakha, M., J. Photochem. Photobiol., A, 2009, vol. 201, nos. 2–3, p. 142. https://doi.org/10.1016/j.jphotochem.2008.10.014
Luo, L., Cooper, A.T., and Fan, M., J. Hazard. Mater., 2009, vol. 161, no. 1, p. 175. https://doi.org/10.1016/j.jhazmat.2008.03.105
Fang, X., Mark, G., and von Sonntag, C., Ultrason. Sonochem., 1996, vol. 3, no. 1, p. 57. https://doi.org/10.1016/1350-4177(95)00032-1
Bubacz, K., Kusiak-Nejman, E., Tryba, B., and Morawski, A.W., J. Photochem. Photobiol., A, 2013, vol. 261, p. 7s. https://doi.org/10.1016/j.jphotochem.2013.04.003
Janus, M., Choina, J., and Morawski, A.W., J. Hazard. Mater., 2009, vol. 166, no. 1, p. 1. https://doi.org/10.1016/j.jhazmat.2008.11.024
Xiao, Q. and Ouyang, L., Chem. Eng. J., 2009, vol. 148, nos. 2–3, p. 248. https://doi.org/10.1016/j.cej.2008.08.024
Matthews, R.W., Radiat. Res., 1980, vol. 83, no. 1, p. 27. https://doi.org/10.2307/3575256
Charbouillot, T., Brigante, M., Mailhot, G., Maddigapu, P.R., Minero, C., and Vione, D., J. Photochem. Photobiol., A, 2011, vol. 222, no. 1, p. 70. https://doi.org/10.1016/j.jphotochem.2011.05.003
Rodríguez, E.M. and von Gunten, U., Water Res., 2020, vol. 177, 115691. https://doi.org/10.1016/j.watres.2020.115691
Henke, A.H., Saunders, T.P., Pedersen, J.A., and Hamers, R.J., Langmuir, 2019, vol. 35, no. 6, p. 2153. https://doi.org/10.1021/acs.langmuir.8b04030
Kisacik, I., Stefanova, A., Ernst, S., and Baltruschat, H., Phys. Chem. Chem. Phys., 2013, vol. 15, no. 13, p. 4616. https://doi.org/10.1039/c3cp44643c
Murugananthan, M., Latha, S.S., Bhaskar Raju, G., and Yoshihara, S., Sep. Purif. Technol., 2011, vol. 79, no. 1, p. 56. https://doi.org/10.1016/j.seppur.2011.03.011
Liu, L., Yang, C., Tan, W., and Wang, Y., ACS Omega, 2020, vol. 5, no. 23, p. 13739. https://doi.org/10.1021/acsomega.0c00903
Kapałka, A., Fóti, G., and Comninellis, C., Electrochem. Commun., 2008, vol. 10, no. 4, p. 607. https://doi.org/10.1016/j.elecom.2008.02.003
García-Osorio, D.A., Jaimes, R., Vazquez-Arenas, J., Lara, R.H., and Alvarez-Ramirez, J., J. Electrochem. Soc., 2017, vol. 164, no. 11, p. E3321. https://doi.org/10.1149/2.0321711jes
Zhang, C., Yang, L., Rong, F., Fu, D., and Gu, Z., Electrochim. Acta, 2012, vol. 64, p. 100. https://doi.org/10.1016/j.electacta.2011.12.122
Lee, Y., Gerrity, D., Lee, M., et al., Environ. Sci. Technol., 2013, vol. 47, no. 11, p. 5872. https://doi.org/10.1021/es400781r
Buxton, G.V. Greenstock, C.L. Helman, W.P. and Ross, A.B., J. Phys. Chem. Ref. Data, 1988, vol. 17, no. 2, p. 513. https://doi.org/10.1063/1.555805
Mandal, S., J. Adv. Oxid. Technol., 2018, vol. 21, no. 1, p. 178. https://doi.org/10.26802/jaots.2017.0075
Murugananthan, M., Yoshihara, S., Rakuma, T., Uehara, N., and Shirakashi, T., Electrochim. Acta, 2007, vol. 52, no. 9, p. 3242. https://doi.org/10.1016/j.electacta.2006.09.073
Martin de Vidales, M.J., Millán, M., Sáez, C., Cañizares, P., and Rodrigo, M.A., Electrochem. Commun., 2016, vol. 67, p. 65. https://doi.org/10.1016/j.elecom.2016.03.014
Zhang, C., Liu, L., Wang, J., Rong, F., and Fu, D., Sep. Purif. Technol., 2013, vol. 107, p. 91. https://doi.org/10.1016/j.seppur.2013.01.033
Rajkumar, D., Palanivelu, K., and Balasubramanian, N., J. Environ. Eng. Sci., 2005, vol. 4, no. 1, p. 1. https://doi.org/10.1139/s04-037
Samarghandi, M.R., Dargahi, A., Shabanloo, A., Nasab, H.Z., Vaziri, Y., and Ansari, A., Arab. J. Chem., 2020, vol. 13, no. 8, p. 6847. https://doi.org/10.1016/j.arabjc.2020.06.038
Tryba, B., Toyoda, M., Morawski, A., Ronaka, R., and Inagaki, M., Appl. Catal., B, 2007, vol. 71, nos. 3–4, p. 163. https://doi.org/10.1016/j.apcatb.2005.12.036
Jiang, Y.L., Liu, H.L., Wang, Q.H., and Jiang, Z.H., J. Environ. Sci. (China), 2006, vol. 18, no. 1, p. 158.
Ishibashi, K., Fujishima, A., Watanabe, T., and Hashimoto, K., Electrochem. Commun., 2000, vol. 2, no. 3, p. 207. https://doi.org/10.1016/S1388-2481(00)00006-0
Okamoto, K., Yamamoto, Y., Tanaka, H., Tanaka, M., and Itaya, A., Bull. Chem. Soc. Jpn., 1985, vol. 58, no. 7, p. 2015. https://doi.org/10.1246/bcsj.58.2015
Peral, J., Casado, j., and Doménech, J., J. Photochem. Photobiol., A, 1988, vol. 44, no. 2, p. 209. https://doi.org/10.1016/1010-6030(88)80093-5
Nakabayashi, Y. and Nosaka, Y., Phys. Chem. Chem. Phys., 2015, vol. 17, no. 45, p. 30570. https://doi.org/10.1039/C5CP04531B
Černigoj, U., Kete, M., and Štangar, U.L., Catal. Today, 2010, vol. 151, nos. 1–2, p. 46. https://doi.org/10.1016/j.cattod.2010.03.043
Newton, G.L. and Milligan, J.R., Radiat. Phys. Chem., 2006, vol. 75, no. 4, p. 473. https://doi.org/10.1016/j.radphyschem.2005.10.011
Peralta, E., Roa, G., Hernandez-Servin, J.A., Romero, R., Balderas, P., and Natividad, R., Electrochim. Acta, 2014, vol. 129, p. 137. https://doi.org/10.1016/j.electacta.2014.02.047
Hoang, N.T., Padan 95 SP treatment by electrochemical process and its combination with other techniques, Doctoral Sci. Dissertation, Chemnitz: Tech. Univ., 2019.
ACKNOWLEDGMENTS
Partial data in this paper were extracted from the doctoral thesis entitled “Padan 95 SP treatment by electrochemical process and its combination with other techniques” completed by the author (Mr. Nguyen Tien Hoang) for the Degree of Doctor of Philosophy at the Technische Universität Chemnitz, Germany [46].
We wish to express our thanks to Professor Rudolf Holze (Chemnitz University of Technology) for his supervision, Professor M. Sommer and Dr. E. Dietzsch (Chemnitz University of Technology) for experimental support and helpful discussions.
Author information
Authors and Affiliations
Contributions
Nguyen Tien Hoang: Writing—original draft, Conceptualization, Methodology, Writing—review & editing, Supervision. Fredrick M. Mwazighe: Writing—review & editing.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Nguyen Tien Hoang, Fredrick M. Mwazighe Effect of Operating Conditions on the Yield of 2-Hydroxyterephthalic Acid for Tracing •OH Radical in Electrochemical Process. Moscow Univ. Chem. Bull. 77, 286–299 (2022). https://doi.org/10.3103/S002713142205008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S002713142205008X