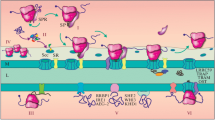
Abstract
Newly synthesized mRNA undergoes several stages of maturation, and the proteins involved in these processes form the mRNP particle. Mature mRNA passes through the nuclear pore from the nucleus to the cytoplasm, where it is transported to the ribosome and mRNA is translated into protein. Recruiting the export and transport factors to the newly synthesized mRNA occurs partly cotranscriptionally. Many of the proteins associate with mRNA from the earliest stages of transcription to its localization in the cytoplasm. Other factors perform their functions strictly at a certain stage. The protein composition of the mRNP particle changes during its adherence to the nuclear pore, passing through it, and being delivered to the site of localization in the cytoplasm. This paper describes the main participants in the export of the mRNP particle, starting with its delivery to the nuclear pore and its attachment to and translocation through the nuclear pore.



Similar content being viewed by others
REFERENCES
Kurshakova, M.M., Georgieva, S.G., and Kopytova, D.V., Protein complexes coordinating mRNA export from the nucleus into the cytoplasm, Mol. Biol. (Moscow), 2016, vol. 50, no. 5, pp. 639–644.
Bjork, P., Persson, J.O., and Wieslander, L., Intranuclear binding in space and time of exon junction complex and NXF1 to premRNPs/mRNPs in vivo, J. Cell Biol., 2015, vol. 211, no. 1, pp. 63–75.
Culjkovic-Kraljacic, B. and Borden, K.L., Aiding and abetting cancer: mRNA export and the nuclear pore, Trends Cell Biol., 2013, vol. 23, no. 7, pp. 328–335.
Monecke, T., Dickmanns, A., and Ficner, R., Allosteric control of the exportin CRM1 unraveled by crystal structure analysis, FEBS J., 2014, vol. 281, no. 18, pp. 4179–4194.
Brennan, C.M., Gallouzi, I., and Steitz, J.A., Protein ligands to HuR modulate its interaction with target mRNAs in vivo, J. Cell Biol., 2000, vol. 151, no. 1, pp. 1–14.
Topisirovic, I., Siddiqui, N., Lapointe, V.L., et al., Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export-competent RNP, EMBO J., 2009, vol. 28, no. 8, pp. 1087–1098.
Yang, J., Bogerd, H.P., Wang, P.J., et al., Two closely related human nuclear export factors utilize entirely distinct export pathways, Mol. Cell, 2001, vol. 8, no. 2, pp. 397–406.
Aitchison, J.D. and Rout, M.P., The yeast nuclear pore complex and transport through it, Genetics, 2012, vol. 190, no. 3, pp. 855–883.
Alber, F., Dokudovskaya, S., Veenhoff, L.M., et al., The molecular architecture of the nuclear pore complex, Nature, 2007, vol. 450, no. 7170, pp. 695–701.
Kiseleva, E., Allen, T.D., Rutherford, S., et al., Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments, J. Struct. Biol., 2004, vol. 145, no. 3, pp. 272–288.
Terry, L.J. and Wente, S.R., Flexible gates: Dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport, Eukaryotic Cell, 2009, vol. 8, no. 12, pp. 1814–1827.
von Appen, A., Kosinski, J., Sparks, L., et al., In situ structural analysis of the human nuclear pore complex, Nature, 2015, vol. 526, no. 7571, pp. 140–143.
Alcazar-Roman, A.R., Tran, E.J., Guo, S., and Wente, S.R., Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export, Nat. Cell Biol., 2006, vol. 8, no. 7, pp. 711–716.
Askjaer, P., Bachi, A., Wilm, M., et al., RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase, Mol. Cell. Biol., 1999, vol. 19, no. 9, pp. 6276–6285.
Bernad, R., van der Velde, H., Fornerod, M., and Pickersgill, H., Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export, Mol. Cell. Biol., 2004, vol. 24, no. 6, pp. 2373–2384.
Hutten, S. and Kehlenbach, R.H., CRM1-mediated nuclear export: To the pore and beyond, Trends Cell Biol., 2007, vol. 17, no. 4, pp. 193–201.
Kehlenbach, R.H., Dickmanns, A., Kehlenbach, A., et al., A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export, J. Cell Biol., 1999, vol. 145, no. 4, pp. 645–657.
Galy, V., Gadal, O., Fromont-Racine, M., et al., Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1, Cell, 2004, vol. 116, no. 1, pp. 63–73.
Vinciguerra, P., Iglesias, N., Camblong, J., et al., Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export, EMBO J., 2005, vol. 24, no. 4, pp. 813–823.
Kosova, B., Pante, N., Rollenhagen, C., et al., Mlp2p, a component of nuclear pore attached intranuclear filaments, associates with nic96p, J. Biol. Chem., 2000, vol. 275, no. 1, pp. 343–350.
Strambio-de-Castillia, C., Blobel, G., and Rout, M.P., Proteins connecting the nuclear pore complex with the nuclear interior, J. Cell Biol., 1999, vol. 144, no. 5, pp. 839–855.
Vinciguerra, P. and Stutz, F., mRNA export: An assembly line from genes to nuclear pores, Curr. Opin. Cell Biol., 2004, vol. 16, no. 3, pp. 285–292.
Casolari, J.M., Brown, C.R., Drubin, D.A., et al., Developmentally induced changes in transcriptional program alter spatial organization across chromosomes, Genes Dev., 2005, vol. 19, no. 10, pp. 1188–1198.
Ma, J., Liu, Z., Michelotti, N., et al., High-resolution three-dimensional mapping of mRNA export through the nuclear pore, Nat. Commun., 2013, vol. 4, p. 2414.
Siebrasse, J.P., Kaminski, T., and Kubitscheck, U., Nuclear export of single native mRNA molecules observed by light sheet fluorescence microscopy, Proc. Natl. Acad. Sci. U. S. A., 2012, vol. 109, no. 24, pp. 9426–9431.
Kiseleva, E., Goldberg, M.W., Allen, T.D., and Akey, C.W., Active nuclear pore complexes in Chironomus: Visualization of transporter configurations related to mRNP export, J. Cell Sci., 1998, vol. 111, part 2, pp. 223–236.
Englmeier, L., Fornerod, M., Bischoff, F.R., et al., RanBP3 influences interactions between CRM1 and its nuclear protein export substrates, EMBO Rep., 2001, vol. 2, no. 10, pp. 926–932.
Fornerod, M., van Deursen, J., van Baal, S., et al., The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88, EMBO J., 1997, vol. 16, no. 4, pp. 807–816.
Forler, D., Rabut, G., Ciccarelli, F.D., et al., RanBP2/Nup358 provides a major binding site for NXF1-p15 dimers at the nuclear pore complex and functions in nuclear mRNA export, Mol. Cell. Biol., 2004, vol. 24, no. 3, pp. 1155–1167.
Tran, E.J., Zhou, Y., Corbett, A.H., and Wente, S.R., The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA: Protein remodeling events, Mol. Cell, 2007, vol. 28, no. 5, pp. 850–859.
Weirich, C.S., Erzberger, J.P., Flick, J.S., et al., Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export, Nat. Cell Biol., 2006, vol. 8, no. 7, pp. 668–676.
Schmitt, C., von Kobbe, C., Bachi, A., et al., Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p, EMBO J., 1999, vol. 18, no. 15, pp. 4332–4347.
Snay-Hodge, C.A., Colot, H.V., Goldstein, A.L., and Cole, C.N., Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export, EMBO J., 1998, vol. 17, no. 9, pp. 2663–2676.
Hodge, C.A., Tran, E.J., Noble, K.N., et al., The Dbp5 cycle at the nuclear pore complex during mRNA export I: dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1, Genes Dev., 2011, vol. 25, no. 10, pp. 1052–1064.
Noble, K.N., Tran, E.J., Alcazar-Roman, A.R., et al., The Dbp5 cycle at the nuclear pore complex during mRNA export II: Nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1, Genes Dev., 2011, vol. 25, no. 10, pp. 1065–1077.
Folkmann, A.W., Noble, K.N., Cole, C.N., and Wente, S.R., Dbp5, Gle1-IP6 and Nup159: A working model for mRNP export, Nucleus (Austin, Tex.), 2011, vol. 2, no. 6, pp. 540–548.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Lazarev
About this article
Cite this article
Glukhova, A.A., Nabirochkina, E.N. & Kopytova, D.V. mRNP Transport in Eukaryotes. mRNP Export from the Nucleus. Mol. Genet. Microbiol. Virol. 33, 182–186 (2018). https://doi.org/10.3103/S0891416818030047
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416818030047