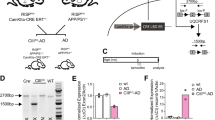
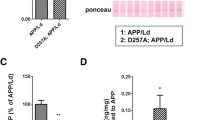
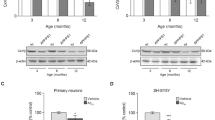
Abstract
Alzheimer’s disease (AD) is an age-related dementia, with the pathological hallmarks of neuritic plaques and neurofibrillary tangles, brain atrophy and loss of synaptic terminals. Dysfunctional mitochondrial bioenergetics is implicated as a contributing factor to the cognitive decline observed in AD. We hypothesized that, in the presence of the AD neurotoxic peptide beta-amyloid, mitochondrial respiration is impaired early in synaptic terminals, which are vital to cognitive performance, preferentially in cognitive centers of the brain. We compared oxygen consumption in synaptosomal and perikaryal mitochondria prepared from the cerebral cortex and cerebellum of wild type (WT) and AD transgenic Tg2576 mice. Compared to WT mice, Tg2576 mice showed decreased mitochondrial respiration in the cerebral cortex specifically in synaptosomal fraction, while the perikaryal mitochondria were unaffected. Neither mitochondrial fraction was affected in the cerebellum of Tg2576 mice as compared to WT. The occurrence of a bioenergetic defect in synaptic terminals of mice overexpressing mutant beta-amyloid, in particular in an area of the brain important to cognition, points to an early role of mitochondrial defects in the onset of cognitive deficits in AD.
Similar content being viewed by others
References
Duara R., Grady C., Haxby J., Sundaram M., Cutler N. R., Heston L., et al., Positron emission tomography in Alzheimer’s disease, Neurology, 1986, 36, 879–887
Haxby J. V., Grady C. L., Duara R., Schlageter N., Berg G., Rapoport S. I., Neocortical metabolic abnormalities precede nonmemory cognitive defects in early Alzheimer’s-type dementia, Arch Neurol, 1986, 43, 882–885
Butterworth R. F., Besnard A. M., Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer’s disease, Metab Brain Dis, 1990, 5, 179–184
Mastrogiacoma F., Lindsay J. G., Bettendorff L., Rice J., Kish S. J., Brain protein and alpha-ketoglutarate dehydrogenase complex activity in Alzheimer’s disease, Ann Neurol, 1996, 39, 592–598
Sorbi S., Bird E. D., Blass J. P., Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain, Ann Neurol, 1983, 13, 72–78
Perry E. K., Perry R. H., Tomlinson B. E., Blessed G., Gibson P. H., Coenzyme A-acetylating enzymes in Alzheimer’s disease: possible cholinergic ‘compartment’ of pyruvate dehydrogenase, Neurosci Lett, 1980, 18, 105–110
Parker W. D., Parks J., Filley C. M., Kleinschmidtdemasters B. K., Electron-Transport Chain Defects in Alzheimers-Disease Brain, Neurology, 1994, 44, 1090–1096
Maurer I., Zierz S., Moller H. J., A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients, Neurobiol Aging, 2000, 21, 455–462
Cottrell D. A., Blakely E. L., Johnson M. A., Ince P. G., Turnbull D. M., Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD, Neurology, 2001, 57, 260–264
Kish S. J., Bergeron C., Rajput A., Dozic S., Mastrogiacomo F., Chang L.J., et al., Brain cytochrome oxidase in Alzheimer’s disease, J Neurochem, 1992, 59, 776–779
Swerdlow R. H., Khan S. M., A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease, Med Hypotheses, 2004, 63, 8–20
Yao J., Irwin R. W., Zhao L., Nilsen J., Hamilton R. T., Brinton R. D., Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease, Proc Natl Acad Sci USA, 2009, 106, 14670–14675
Dragicevic N., Mamcarz M., Zhu Y. Y., Buzzeo R., Tan J., Arendash G. W., Bradshaw P. C., Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice, J Alzheimers Dis, 2010, 20, S535–S550
Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., et al., Correlative memory deficits, A beta elevation, and amyloid plaques in transgenic mice, Science, 1996, 274, 99–102
Brown M. R., Sullivan P. G., Geddes J. W., Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria, J Biol Chem, 2006, 281, 11658–11668
Choi S. W., Gerencser A.A., Nicholls D. G., Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem, 2009, 109, 1179–1191
Wang J., Ho L., Zhao W., Ono K., Rosensweig C., Chen L., et al., Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease, J Neurosci, 2008, 28, 6388–6392
Du H., Guo L., Yan S., Sosunov A. A., McKhann G. M., Yan S. S., Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model, Proc Natl Acad Sci USA, 2010, 107, 18670–18675
Hansson Petersen C. A., Alikhani N., Behbahani H., Wiehager B., Pavlov P. F., Alafuzoff I., et al., The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae, Proc Natl Acad Sci USA, 2008, 105, 13145–13150
Devi L., Prabhu B. M., Galati D. F., Avadhani N. G., Anandatheerthavarada H. K., Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction, J Neurosci, 2006, 26, 9057–9068
Crouch P. J., Blake R, Duce J. A., Ciccotosto G.D., Li Q.X., Barnham K.J., et al., Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta 1–42, J Neurosci, 2005, 25, 672–679
Manczak M., Anekonda T.S., Henson E., Park B.S., Quinn J., Reddy P.H., Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression, Hum Mol Genet, 2006, 15, 1437–1449
Lesne S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A., et al., A specific amyloid-beta protein assembly in the brain impairs memory, Nature, 2006, 440, 352–357
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Varghese, M., Zhao, W., Wang, J. et al. Mitochondrial bioenergetics is defective in presymptomatic Tg2576 AD Mice. Translat.Neurosci. 2, 1–5 (2011). https://doi.org/10.2478/s13380-011-0011-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s13380-011-0011-8