Abstract
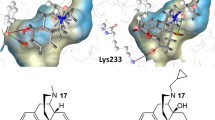
An opioid receptor like (ORL1) receptor is one of a family of G-protein-coupled receptors (GPCR); it represents a new pharmaceutical target with extensive therapeutic potential for the regulation of important biological functions such as nociception, mood disorders, drug abuse, learning or cardiovascular control. Although the crystal structure of the inactive form of the ORL1 receptor has been determined, little is known about its activation. By using X-ray structures of the β2-adrenegic receptor in its inactive (2RH1) and active (3P0G) states as templates, inactive and active homology models of the ORL1 receptor were constructed. Structurally diverse sets of strongly binding antagonists and agonists were docked with both ORL1 receptor forms. The major receptor-ligand interactions responsible for antagonist and agonist binding were identified. Although both sets of ligands, agonists and antagonists, bind to the same region of the receptor, they occupy partially different binding pockets. Agonists bind to the inactive receptor in a slightly different manner than antagonists. This difference is more pronounced in binding to the active ORL1 receptor model and points to the amino acids at the extracellular end of TM6, suggesting that this region is important for receptor-activation.
Similar content being viewed by others
References
Accelrys Software (2005). Insight II [computer software]. San Diego, CA, USA: Accelrys Software.
Audet, M., & Bouvier, M. (2008). Insights into signaling from the β2-adrenergic receptor structure. Nature Chemical Biology, 4, 397–403. DOI: 10.1038/nchembio.97.
Avogadro (2013). An open-source molecular builder and visualization tool, version 1.0.0. [computer software]. Retrieved on October 7, 2013 from: http://avogadro.Openmolecules.net/
Ballesteros, J. A., & Weinstein, H. (1995). Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G-proteincoupled receptors. Methods in Neurosciences, 25, 366–428. DOI: 10.1016/s1043-9471(05)80049-7.
Bigoni, R., Rizzi, D., Rizzi, A., Camarda, V., Guerrini, R., Lambert, D. G., Hashiba, E., Berger, H., Salvadori, S., Regoli, D., & Calo’, G. (2002). Pharmacological characterization of [(pX)Phe4]nociceptin(1–13)amide analogues. Naunyn-Schmiedeberg’s Archives of Pharmacology, 365, 442–449. DOI: 10.1007/s00210-002-0548-8.
Bröer, B. M., Gurrath, M., & Höltje, H. D. (2003). Molecular modelling studies on the ORL1-receptor and ORL1-agonists. Journal of Computer-Aided Molecular Design, 17, 739–754. DOI: 10.1023/b:jcam.0000017491.97244.69.
Cherezov, V., Rosenbaum, D. M., Hanson, M. A., Rasmussen, S. G. F., Thian, F. S., Kobilka, T. S., Choi, H. J., Kuhn, P., Weis, W. I., Kobilka, B. K., & Stevens, R. C. (2007). High-resolution crystal structure of an engineered human β2-adrenergic G-protein-coupled receptor. Science, 318, 1258–1265. DOI: 10.1126/science.1150577.
Daga, P. R., & Zaveri, N. T. (2012). Homology modeling and molecular dynamics simulations of the active state of the nociceptin receptor reveal new insights into agonist binding and activation. Proteins: Structure, Function, and Bioinformatics, 80, 1948–1961. DOI: 10.1002/prot.24077.
Goujon, M., McWilliam, H., Li, W. H., Valentin, F., Squizzato, S., Paern, J., & Lopez, R. (2010). A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Research, 38, W695–W699. DOI: 10.1093/nar/gkq313.
Guerrini, R., Calo’, G., Rizzi, A., Bianchi, C., Lazarus, L. H., Salvadori, S., Temussi, P. A., & Regoli, D. (1997). Address and message sequences for the nociceptin receptor: A structure-activity study of nociceptin-(1-13)-peptide amide. Journal of Medicinal Chemistry, 40, 1789–1793. DOI: 10.1021/jm970011b.
Hanwell, M. D., Curtis, D. E., Lonie, D. C., Vandermeersch, T., Zurek, E., & Hutchison, G. R. (2012). Avogadro: An advanced semantic chemical editor, visualization and analysis platform. Journal of Cheminformatics, 4, 17. DOI: 10.1186/1758-2946-4-17.
Huang, X. Q., Jiang, H. L., Luo, X. M., Chen, K. X., Zhu, Y. C., Ji, R. Y., & Cao, Y. (2000). Comparative molecular modeling on 3D-structure of opioid receptor-like 1 receptor. Acta Pharmacologica Sinica, 21, 529–535.
Humphrey, W., Dalke, A., & Schulten, K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics, 14, 33–38. DOI: 10.1016/0263-7855(96)00018-5.
Isozaki, K., Okada, K., Koikawa, S., Nose, T., Costa, T., & Shimohigashi, Y. (2006). Residual roles of hydrophobic amino acids in the fifth transmembrane domain of ORL1 receptor in its activation. Peptide Science, 43, 11.
Isozaki, K., Li, J. L., Okada, K., Nishimura, H., Matsushima, A., Nose, T., Costa, T., & Shimohigashi, Y. (2009). Spare interactions of highly potent [Arg14, Lys15] nociceptin for cooperative induction of ORL1 receptor activation. Bioorganic & Medicinal Chemistry, 17, 7904–7908. DOI: 10.1016/j.bmc.2009.10.026.
Lambert, D. G. (2008). The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. X. Nature Reviews: Drug Discovery, 7, 694–710. DOI: 10.1038/nrd2572.
Laskowski, R. A., MacArthur, M. W., Moss, D. S., & Thornton, J. M. (1993). PROCHECK: A program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26, 283–291. DOI: 10.1107/s0021889892009944.
Liu, M., He, L., Hu, X. P., Liu, P. Q., & Luo, H. B. (2010). 3D-QSAR, homology modeling and molecular docking studies on spiropiperidines analogues as agonists of nociceptin/orphanin FQ receptor. Bioorganic & Medicinal Chemistry Letters, 20, 7004–7010. DOI: 10.1016/j.bmcl.2010.09.116.
Meng, F., Taylor, L. P., Hoversten, M. T., Ueda, Y., Ardati, A., Reinscheid, R. K., Monsma, F. J., Watson, S. J., Civelli, O., & Akil, H. (1996). Moving from the orphanin FQ receptor to an opioid receptor using four point mutations. Journal of Biological Chemistry, 271, 32016–32020. DOI: 10.1074/jbc.271.50.32016.
Mollereau, C., Mouledous, L., Lapalu, S., Cambois, G., Moisand, C., Butour, J. L., & Meunier, J. C. (1999). Distinct mechanisms for activation of the opioid receptor-like 1 and κ-opioid receptors by nociceptin and dynorphin A. Molecular Pharmacology, 55, 324–331. DOI: 10.1124/mol.55.2.324.
Momany, F. A., & Rone, R. (1992). Validation of the general purpose QUANTA ®3.2/CHARMm®force field. Journal of Computational Chemistry, 13, 888–900. DOI: 10.1002/jcc.54013074.
Moulédous, L., Topham, C. M., Moisand, C., Mollereau, C., & Meunier, J. C. (2000). Functional inactivation of the nociceptin receptor by alanine substitution of glutamine 286 at the C terminus of transmembrane segment VI: Evidence from a site-directed mutagenesis study of the ORL1 receptor transmembrane-binding domain. Molecular Pharmacology, 57, 495–502. DOI: 10.1124/mol.57.3.495.
Mustazza, C., Borioni, A., Sestili, I., Sbraccia, M., Rodomonte, A., Farretti, R., & Del Giudice, M. R. (2006). Synthesis and evaluation as NOP ligands of some spiro[piperidine-4,2′(1′H)-quinazolin]-4′(3′H)-ones and spiro[piperidine-4,5′(6′H)-[1,2, 4]triazolo[1,5-c]quinazolines]. Chemical & Pharmaceutical Bulletin, 54, 611–622. DOI: 10.1248/cpb.54.611.
Okano, M., Mito, J., Maruyama, Y., Masuda, H., Niwa, T., Nakagawa, S., Nakamura, Y., & Matsuura, A. (2009). Discovery and structure-activity relationships of 4-aminoquinazoline derivatives, a novel class of opioid receptor like-1 (ORL1) antagonists. Bioorganic & Medicinal Chemistry, 17, 119–132. DOI: 10.1016/j.bmc.2008.11.012.
Philip, A. E., Poupaert, J. H., & McCurdy, C. R. (2005). Opioid receptor-like 1 (ORL1) molecular “Road Map” to understanding ligand interaction and selectivity. Current Topics in Medicinal Chemistry, 5, 325–340. DOI: 10.2174/1568026053544489.
Rasmussen, S. G. F., Choi, H. J., Fung, J. J., Pardon, E., Casarosa, P., Chae, P. S., DeVree, B. T., Rosenbaum, D.M., Thian, F. S., Kobilka, T. S., Schnapp, A., Konetzki, I., Sunahara, R. K., Gellman, S. H., Pautsch, A., Steyaert, J., Weis, W. I., & Kobilka, B. K. (2011). Structure of a nanobodystabilized active state of the β2-adrenoceptor. Nature, 469, 175–180. DOI: 10.1038/nature09648.
Riadi, G. (2006, June 21). Average structure and RMSD. Message posted on VMD-L mailing list, archived at http://www.ks.uiuc.edu/Research/vmd/mailinglist/vmd-l/7251.html
Rosenbaum, D. M., Zhang, C., Lyons, J. A., Holl, R., Aragao, D., Arlow, D. A., Rasmussen, S. G. F., Choi, H. J., DeVree, B. T., Sunahara, R. K., Chae, P. S., Gellman, S. H., Dror, R. O., Shaw, D. E., Weis, W. I., Caffrey, M., Gmeiner, P., & Kobilka, B. K. (2011). Structure and function of an irreversible agonist-β2-adrenoceptor complex. Nature, 469, 236–240. DOI: 10.1038/nature09665.
Šali, A., & Blundell, T. L. (1993). Comparative protein modeling by satisfaction of spatial restraints. Journal of Molecular Biology, 234, 779–815. DOI: 10.1006/jmbi.1993.1626.
Schmidt, M. W., Baldridge, K. K., Boatz, L. A., Elbert, S. T., Gordon, M. S., Jensen, J. H., Koseki, S., Matsunaga, N., Nguyen, K. A., Su, S. J., Windus, T. L., Dupuis, M., & Montgomery, J. A. (1993). General atomic and molecular electronic structure system. Journal of Computational Chemistry, 14, 1347–1363. DOI: 10.1002/jcc.540141112.
Schneider, M., Wolf, S., Schlitter, J., & Gerwert, K. (2011). The structure of active opsin as a basis for identification of GPCR agonists by dynamic homology modelling and virtual screening assays. FEBS Letters, 585, 3587–3592. DOI: 10.1016/j.febslet.2011.10.027.
Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W. Z., Lopez, R., McWilliam, H., Remmert, M., Söding, J., Thompson, J. D., & Higgins, D. G. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7, 539. DOI: 10.1038/msb.2011.75.
Stewart, J. J. P. (2007). Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. Journal of Molecular Modeling, 13, 1173–1213. DOI: 10.1007/s00894-007-0233-4.
Stewart, J. P. P. (2008). MOPAC2009 [computer software]. Colorado Springs,CO, USA: Stewart Computational Chemistry.
The UniProt Consortium (2014). Activities at the universal protein resource (UniProt). Nucleic Acids Research, 42, D191–D198. DOI: 10.1093/nar/gkt1140.
Thompson, A. A., Liu, W., Chun, E., Katritch, V., Wu, H. X., Vardy, E., Huang, X. P., Trapella, C., Guerrini, R., Calo, G., Roth, B. L., Cherezov, V., & Stevens, R. C. (2012). Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature, 485, 395–399. DOI: 10.1038/nature11085.
Topham, C. M., Mouledous, L., Poda, G., Maigret, B., & Meunier, J. C. (1998). Molecular modeling of the ORL1 receptor and its complex with nociceptin. Protein Engineering Design and Selection, 11, 1163–1179. DOI: 10.1093/protein/11.12.1163.
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. Journal of Computational Chemistry, 31, 455–461. DOI: 10.1002/jcc.21334.
Trzaskowski, B., Latek, D., Yuan, S., Ghoshdastider, U., Debinski, A., & Filipek, S. (2012). Action of molecular switches in GPCRs — theoretical and experimental studies. Current Medicinal Chemistry, 19, 1090–1109. DOI: 10.2174/092986712799320556.
Wacker, D., Fenalti, G., Brown, M. A., Katritch, V., Abagyan, R., Cherezov, V., & Stevens, R. C. (2010). Conserved binding mode of human β2-adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. Journal of the American Chemical Society, 132, 11443–11445. DOI: 10.1021/ja105108q.
Yoshizumi, T., Miyazoe, H., Ito, H., Tsujita, T., Takahashi, H., Asai, M., Ozaki, S., Ohta, H., & Okamoto, O. (2008). Design, synthesis and structure-activity relationship study of a novel class of ORL1 receptor antagonists based on N-biarylmethyl spiropiperidine. Bioorganic & Medicinal Chemistry Letters, 18, 3778–3782. DOI: 10.1016/j.bmcl.2008.05.036.
Zaveri, N., Jiang, F. M., Olsen, C., Polgar, W., & Toll, L. (2005). Small-molecule agonists and antagonists of the opioid receptor-like receptor (ORL1, NOP): Ligand-based analysis of structural factors influencing intrinsic activity at NOP. The AAPS Journal, 7, E345–352. DOI: 10.1208/aapsj070234.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Senćanski, M., Ivanović, M.D. & Došen-Mićović, L. Modelling of ORL1 receptor-ligand interactions. Chem. Pap. 68, 1305–1316 (2014). https://doi.org/10.2478/s11696-014-0577-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0577-z