Summary
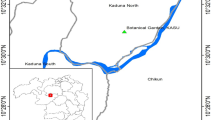
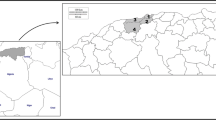
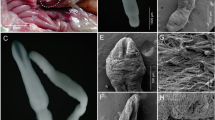
Using a sedimentation method, the prevalence of the nodular worm Oesophagostomum stephanostomum (Nematoda: Strongylida) in western lowland gorillas at Moukalaba-Doudou National Park (MDNP), Gabon, was determined in fecal samples collected between January 2007 and October 2011, along with their coprocultures. Concurrently, possible zoonotic Oesophagostomum infections in villagers living near MDNP were assessed from their fecal samples collected during October and November of 2011. In the gorillas, strongylid (Oesophagostomum and/or hookworm) eggs were found in 47 of 235 fecal samples (20.0 %) and Oesophagostomum larvae were detected in 101 of 229 coprocultures (44.1 %). In the villagers, strongylid eggs were found in 9 of 71 fecal samples (12.7 %), but no Oesophagostomum larvae were detected in coprocultures. The internal transcribed spacer (ITS) region of ribosomal RNA gene (rDNA) and cytochrome c oxidase subunit-1 (cox-1) region of mitochondrial DNA (mtDNA) of coprocultured Oesophagostomum larvae were amplified using parasite DNA extracted from 7–25 larvae/sample, cloned into Escherichia coli, and sequenced. Sequenced rDNA contained 353/354-bp long ITS1, 151-bp long 5.8S rDNA, and 227-bp long ITS2. Parts of clones showed variations at 1–3 bases in the ITS1 region at a frequency of 24/68 (35.3 %) and at 1–2 bases in the ITS2 region at a frequency of 7/68 (10.3 %), whereas the 5.8S rDNA was essentially identical. Sequenced cox-1 gene of the parasites, 849 bp in length, showed a higher number of nucleotide variations, mainly at the third nucleotide position of the codon. The majority of clones (27/41 (65.9 %)) had an identical amino acid sequence. These results suggest that at MDNP, Gabon, only a single population of O. stephanostomum with a degree of genetic diversity is prevalent in western lowland gorillas, without zoonotic complication in local inhabitants. The possible genetic variations in the ITS region of rDNA and cox-1gene of mtDNA presented here may be valuable when only a limited amount of material is available for the molecular species diagnosis of O. stephanostomum.
Similar content being viewed by others
References
Anderson, R. C. (1992): Nematode Parasites of Vertebrates; their Development and Transmission. Oxon, Wallingford, UK, CAB International, 578 pp.
Ando, C., Iwata, Y., Yamagiwa, J. (2008): Progress of habituation of western lowland gorillas and their reaction to observers in Moukalaba-Doudou National Park, Gabon. Afr. Study Monogr. Suppl., 39: 55–69
Bezjian, M., Gillespie, T. R., Chapman, C. A., Grenier, E. C. (2008): Coprologic evidence of gastrointestinal helminths of forest baboons, Papio anubis, in Kibale National Park, Uganda. J. Wildl. Dis., 44: 878–887
Blotkamp, J., Krepel, H. P., Kumar, V., Baeta, S., Van’t Noordende, J. M., Polderman, A. M. (1993): Observation on the morphology of adult and larval stages of Oesophagostomum sp. isolated from man in northern Togo and Ghana. J. Helminthol., 67: 49–61
Chabaud, A. G., Durette-desset, M.-C. (1974): Description d’un nouveau Nématode Oesophagostome, parasite d’Hyemoschus au Gabon, et remarques sur le genre Oesophagostomum. Bull. Mus. Natl. Hist. Nat. Paris, 184: 1415–1424
Chabaud, A. G., Lariviere, M. (1958): Sur les oesophagostomes parasites de l’homme. Bull. Soc. Pathol. Exot., 51: 384–393
de Gruijter, J. M., Blotkamp, J., Gasser, R. B., Polderman, A. M. (2006): Morphological variability within Oesophagostomum bifurcum among different primate species from Ghana. J. Helminthol., 80: 357–361
de Gruijter, J. M., Gasser, R. B., Polderman, A. M., Asigri, V., Dijkshoorn, L. (2005): High resolution DNA fingerprinting by AFLP to study the genetic variation among Oesophagostomum bifurcum (Nematoda) from human and non-human primates from Ghana. Parasitology, 130: 229–237
de Gruijter, J. M., Polderman, A. M., Zhu, X. Q., Gasser, R. B. (2002): Screening for haplotypic variability within Oesophagostomum bifurcum (Nematoda) employing a single-strand conformation polymorphism approach. Mol. Cell. Probes, 16: 185–190
de Gruijter, J. M., Ziem, J., Verweij, J. J., Polderman, A. M., Gasser, R. B. (2004): Genetic substructuring within Oesophagostomum bifurcum (Nematoda) from human and non-human primates from Ghana based on random amplified polymorphic DNA analysis. Am. J. Trop. Med. Hyg. 71: 227–233
File, S. K., Mcgrew, W. C., Tutin, C. E. G. (1976): The intestinal parasites of a community of feral chimpanzees, Pan troglodytes schweinfurthii. J. Parasitol., 62: 259–261
Freeman, A. S., Kinsella, J. M., Cipolletta, C., Deem, S. L., Karesh, W. B. (2004): Endoparasites of western lowland gorillas (Gorilla gorilla gorilla) at Bai Hokou, Central African Republic. J. Wildl. Dis., 40: 775–781
Gasser, R. B., Woods, W. G., Blotkamp, C., Verweij, J., Storey, P. A., Polderman, A. M. (1999a): Screening for nucleotide variations in ribosomal DNA arrays of Oesophagostomum bifurcum by polymerase chain reactioncoupled single-strand conformation polymorphism. Electrophoresis, 20: 1486–1491
Gasser, R. B., Woods, W. G., Huffman, M. A., Blotkamp, J., Polderman, A. M. (1999b): Molecular separation of Oesophagostomum stephanostomum and Oesophagostomum bifurcum (Nematoda: Strongyloidea) from non-human primates. Int. J. Parasitol., 29: 1087–1091
Gasser, R. B., de Gruijter, J. M., Polderman, A. M. (2006): Insights into the epidemiology and genetic makeup of Oesophagostomum bifurcum from human and nonhuman primates using molecular tools. Parasitology, 132: 453–460
Gasser, R. B., de Gruijter, J. M., Polderman, A. M. (2009): The utility of molecular methods for elucidating primate-pathogen relationships — the Oesophagostomum bifurcum example. In: Huffman, M. A., Chapman, C. A. (Eds) Primate Parasite Ecology: The Dynamics and Study of Host-Parasite Relationships. Cambridge, UK: Cambridge University Press, pp. 47–62
Ghai, R. R., Chapman, C. A., Omeja, P. A., Davies, J., Goldberg, T. L. (2014): Nodular worm infection in humans and wild primates in Uganda: cryptic species in newly identified region of human transmission. PLos Negl. Trop. Dis., 8: e2541. DOI: 10.1371/journal.pntd.0002641
Gottschling, M., Plötner, J. (2004): Secondary structure models of the nuclear internal transcribed spacer regions and 5.8S rRNA in Calciodinelloideae (Peridiniaceae) and other dinoflagellates. Nucleic Acids Res., 32: 307–315
Gruber, A. R., Lorenz, R., Bernhart, S. H., Neuböck, R., Hofacker, I. L. (2008): The Vienna RNA Websuite. Nucleic Acids Res., 36(Web Server issue): W70–W74
Hasegawa, H. (2009a): Methods of collection and identification of minute nematodes from the feces of primates, with special application to coevolutionary study of pinworms. In: HUFFMAN, M. A., CHAPMAN, C. A. (Eds) Primate Parasite Ecology: The Dynamics and Study of Host-Parasite Relationships. Cambridge, UK: Cambridge University Press, pp. 29–46
Hasegawa, H. (2009b): Useful diagnostic references and images of protozoans, helminths, and nematodes commonly found in wild primates. In: Huffman, M. A., Chapman, C. A. (Eds) Primate Parasite Ecology: The Dynamics and Study of Host-Parasite Relationships. Cambridge, UK: Cambridge University Press, pp. 507–513
Hasegawa, H., Sato, H., Fujita, S., Nguema, P. P., Nobusue, K., Miyagi, K., Kooriyama, T., Takenoshita, Y., Noda, S., Sato, A., Morimoto, A., Ikeda, Y., Nishida, T. (2010): Molecular identification of causative agent of human strongyloidiasis acquired in Tanzania: dispersal and diversity of Strongyloides spp. and their host. Parasitol. Int., 59: 407–413
Hofacker, I. L. (2003): Vienna RNA secondary structure server. Nucleic Acid Res., 31: 3429–3431
Huffman, M. A., Caton, J. M. (2001): Self-induced increase of gut motility and the control of parasitic infections in wild chimpanzees. Int. J. Primatol., 22: 329–346
Huffman, M. A., Page, J. E., Sukhdeo, M. V. K., Gotoh, S., Kalunde, M. S., Chandrasiri, T., Towers, G. H. N. (1996): Leaf-swallowing by chimpanzees, a behavioral adaptation for the control of strongyle nematode infections. Int. J. Primatol., 17: 475–503
Huffman, M. A., Gotoh, S., Turner, L. A., Hamai, M., Yoshida, K. (1997): Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates, 38: 111–125
Hwang, S.-K., Kim, J.-G. (2000): Secondary structural and phylogenetic implications of nuclear large subunit ribosomal RNA in the ectomycorrhizal fungus Tricholoma matsutake. Curr. Microbiol., 40: 250–256
Jex, A. R., Hall, R. S., Littlewood, D. T., Gasser, R. B. (2010): An integrated pipeline for next-generation sequencing and annotation of mitochondrial genomes. Nucleic Acids Res., 38: 522–533
Krief, S., Huffman, M. A., Sévenet, T., Guillot, J., Bories, C., Hladik, C. M., Wrangham, R. W. (2005): Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int. J. Primatol., 26: 467–490
Krief, S., Jamart, A., Mahé, S., Leendertz, F. H., Mätz-Rensing, K., Crespeau, F., Bain, O., Guillot, J. (2008): Clinical and pathologic manifestation of oesophagostomosis in African great apes: does self-medication in wild apes influence disease progression?. J. Med. Primatol., 37: 188–195
Lichtenfels, J. R. (1980) No. 7. Keys to Genera of the Superfamily Strongyloidea. In: Anderson, R. C., Chabaud, A. G., Willmott, S. (Eds) CIH Keys to the Nematode Parasites of Vertebrates. Oxon, UK: CAB International, pp. 1–41
Lin, R.-Q., Liu, G.-H., Hu, M., Song, H.-Q., Wu, X.-Y., Li, M.-Q., Zhang, Y., Zou, F.-C., Zhu, X.-Q. (2012a): Oesophagostomum dentatum and Oesophagostomum quadrispinulatum: characterization of the complete mitochondrial genome sequences of the two pig nodule worms. Exp. Parasitol., 131: 1–7
Lin, R.-Q., Liu, G.-H., Song, H.-Q., Zhang, Y., Li, M.-W., Zou, F.-C., Yuan, Z.-G., Weng, Y.-B., Zhu, X.-Q. (2012b): Sequence variability in three mitochondrial genes between the two pig nodule worms Oesophagostomum dentatum and O. quadrispinulatum. Mitochondr. DNA, 23: 182–186
Makouloutou, P., Setsuda, A., Yokoyama, M., Tsuji, T., Saita, E., Torii, H., Kaneshiro, Y., Sasaki, M., Maeda, K., Une, Y., Hasagawa, H., Sato, H. (2013): Genetic variation of Gongylonema pulchrum from wild animals and cattle in Japan based on ribosomal RNA and mitochondrial cytochrome c oxidase subunit I genes. J. Helminthol., 87: 326–335
Newton, L. A., Chilton, N. B., Beveridge, I., Gasser, R. B. (1998): Systematic relationships of some members of the genera Oesophagostomum and Chabertia (Nematoda: Chabertiidae) based on ribosomal DNA sequence data. Int. J. Parasitol., 28: 1781–1789
Pit, D. S. S., Esther, F. E. M., Raspoort, E. C., Baeta, S. M., Polderman, A. M. (1999): Geographic distribution and epidemiology of Oesophagostomum bifurcum and hookworm infections in humans in Togo. Am. J. Trop. Med. Hyg., 61: 951–955
Polderman, A. M., Blotkamp, J. (1995): Oesophagostomum infection in humans. Parasitol. Today, 11: 451–456
Romstad, A., Gasser, R. B., Monti, J. R., Polderman, A. M., Nansen, P., Pit, D. S., Chilton, N. B. (1997): Differentiation of Oesophagostomum bifurcum from Necator americanus by PCR using genetic markers in spacer ribosomal DNA. Mol. Cell. Probes, 11: 169–176
Sato, H., Suzuki, K., Osanai, A., Kamiya, H., Furuoka, H. (2006): Identification and characterization of the threadworms, Strongyloides procyonis, from feral raccoons (Procyon lotor) in Japan. J. Parasitol., 92: 63–68
Stewart, T. B., Gasbarre, L. C. (1989): The veterinary importance of nodular worms (Oesophagostomum spp.). Parasitol. Today, 5: 209–213
Teacher, A. G. F., Griffiths, D. J. (2011): HapStar: automated haplotype network layout and visualization. Mol. Ecol. Resour., 11: 151–153
Thompson, J. D., Higgins, D. G., Gibson, T. J. (1994): CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22: 4673–4680
van Lieshout, L., De Fruijter, J. M., Adu-nsiah, M., Haizel, M., Verweij, J. J., Brienen, E. A., Gasser, R. B., Polderman, A. M. (2005): Oesophagostomum bifurcum in non-human primates is not a potential reservoir for human infection in Ghana. Trop. Med. Int. Health, 10: 1315–1320
van Wyk, J. A., Cabaret, J., Michael, L. M. (2004): Morphological identification of nematode larvae of small ruminants and cattle simplified. Vet. Parasitol., 119: 277–306
Yelifari, L., Bloch, P., Magnussen, P., Van Lieshout, L., Dery, G., Anemana, S., Agongo, E., Polderman, A. M. (2005): Distribution of human Oesophagostomum bifurcum, hookworm and Strongyloides stercoralis infections in northern Ghana. Trans. Roy. Soc. Trop. Med. Hyg., 99: 32–38
Zuker, M. (2003): Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res., 31: 3406–3415
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Makouloutou, P., Mbehang Nguema, P.P., Fujita, S. et al. Prevalence and genetic diversity of Oesophagostomum stephanostomum in wild lowland gorillas at Moukalaba-Doudou National Park, Gabon. Helminthologia 51, 83–93 (2014). https://doi.org/10.2478/s11687-014-0214-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11687-014-0214-y