Abstract
Purpose
Free-living amoeba (FLA) including Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria are among the soil-born parasites. There are reports of FLA-related keratitis with a history of contact with soil and dust sources, particularly among the farmers. Due to lack of the previous studies on the farmland soils and a limited number of researches conducted on recreational soils in Iran, the present study was conducted.
Methods
A total of 93 soil samples including farming lands and recreational places were tested for the presence of Acanthamoeba spp. Balamuthia mandrillaris, Naegleria, and Vermamoeba using morphological key and sequencing-based tools. Pathogenicity of Acanthamoeba positive strains was also evaluated. To verify genetic associations and taxonomic status of isolated amoeba, a phylogenetic tree was built by MEGA 5.05 software inferred by the 18S rRNA gene based on maximum likelihood algorithm.
Results
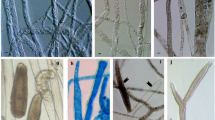
Overall, 28 samples (30%) were contaminated with potentially pathogenic FLA, and according to the sequencing data, 17 strains were successfully sequenced. The isolated Acanthamoeba belonged to T2, T4, T5, mixed T4 and T5 contaminations, and T11. ITS sequencing revealed the occurrence of one strain of Naegleria canariensis. Four strains of Vermamoeba vermiformis were also confirmed. Morphological survey and PCR assay failed to show any positive results for Balamuthia mandrillaris. Pathogenic potential of the Acanthamoeba strains showed that T2, T4, and T11 genotypes were highly pathogenic, whereas T5 genotypes demonstrated lower pathogenic potential.
Conclusion
The results indicate that soil could be a serious hazard to human health, and therefore, further studies are expected to investigate the source of infection in patients developing FLA-related diseases. The present study is the first to investigate FLA in the farmland soils in Iran and the first to report the presence of N. canariensis in the country.



Similar content being viewed by others
References
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp as agents of disease in humans. Clin Microbiol Rev 16(2):273–307
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50(1):1–26
Javanmard E, Niyyati M, Lorenzo-Morales J, Lasjerdi Z, Behniafar H, Mirjalali H (2017) Molecular identification of waterborne free living amoebae (Acanthamoeba, Naegleria and Vermamoeba) isolated from municipal drinking water and environmental sources, Semnan province, north half of Iran. Exp Parasitol 183:240–244
Nazar M, Haghighi A, Niyyati M, Eftekhar M, Tahvildar-Biderouni F, Taghipour N, Abadi A, Mojarad EN, Athari A (2011) Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. J Water Health 9(3):603–608
Solgi R, Niyyati M, Haghighi A, Taghipour N, Tabaei SJS, Eftekhar M, Nazemalhosseini Mojarad E (2012) Thermotolerant Acanthamoeba spp. isolated from therapeutic hot springs in Northwestern Iran. J Water Health 10(4):650–656
Khan NA, Siddiqui R (2009) Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int J Parasitol 39(14):1611–1616
Chan L-L, Mak J-W, Low Y-T, Koh T-T, Ithoi I, Mohamed SM (2011) Isolation and characterization of Acanthamoeba spp. from air-conditioners in Kuala Lumpur. Malaysia Acta Trop 117(1):23–30
Üstüntürk-Onan M, Walochnik J (2018) Identification of free-living amoebae isolated from tap water in Istanbul. Turkey Exp Parasitol 195:34–37
Basher MHA, Ithoi I, Mahmud R, Abdulsalam AM, Foead AI, Dawaki S, Atroosh WMM, Nissapatorn V, Abdullah WO (2018) Occurrence of Acanthamoeba genotypes in Central West Malaysian environments. Acta Trop 178:219–228
Daryani A, Sharif M, Gholami S, Dodangeh S, Moghddam Y, Sarvi S, Montazeri M (2018) Acanthamoeba spp. from water and soil sources in Iran: a systematic review and meta-analysis. Ann Parasitol 64(4):285–297
Niyyati M, Rezaeian (2015) Current status of Acanthamoeba in Iran: a narrative review article. Iran J Parasitol 10(2):157–163
Rezaeian M, Niyyati M, Farnia S, Haghi AM (2008) Isolation of Acanthamoeba spp. from different environmental sources. Iran J Parasitol 3(1):44–47
Niyyati M, Ebrahimi M, Haghighi A, Haydari S (2013) Isolation and genotyping of Acanthamoeba spp. from recreational soil of parks in Tehran, Iran. Armaghane Danesh 18(7):530–538
Meighani M, Eslamirad Z, Hajihossein R, Ahmadi A, Saki S (2018) Isolation and genotyping of Acanthamoeba from soil samples in Markazi Province, Iran. Open Access Maced J Med Sci 6(12):2290
Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohebali M, Maghsood AH, Farnia S, Valladares B, Rezaeian M (2010) First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol 126(1):89–90
Abedkhojasteh H, Niyyati M, Rahimi F, Heidari M, Farnia S, Rezaeian M (2013) First report of Hartmannella keratitis in a cosmetic soft contact lens wearer in Iran. Iran J Parasitol 8(3):481
Hajialilo E, Niyyati M, Solaymani M, Rezaeian M (2015) Pathogenic free-living amoebae isolated from contact lenses of keratitis patients. Iran J Parasitol 10(4):541
Movahedi Z, Shokrollahi MR, Aghaali M, Heydari H (2012) Primary amoebic meningoencephalitis in an Iranian Infant. Case Rep Med 2012:782854
Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, Valladares B (2009) Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res 106(1):279–281
Niyyati M, Karamati SA, Morales JL, Lasjerdi Z (2016) Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol Res 115(2):541–545
Latifi A, Niyyati M, Lorenzo-Morales J, Haghighi A, Tabaei SS, Lasjerdi Z (2016) Presence of Balamuthia mandrillaris in hot springs from Mazandaran province, northern Iran. Epidemiol Infect 144(11):2456–2461
Latifi AR, Niyyati M, Lorenzo-Morales J, Haghighi A, Tabaei SJS, Lasjerdi Z, Azargashb E (2017) Occurrence of Naegleria species in therapeutic geothermal water sources, Northern Iran. Acta Parasitol 62(1):104–109
Lasjerdi Z, Niyyati M, Haghighi A, Shahabi S, Biderouni FT, Taghipour N, Eftekhar M, Mojarad EN (2011) Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol Res 109(3):575–580
Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ (2001) Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. Eur J Clin Microbiol Infect 39(5):1903–1911
Pélandakis M, Pernin P (2002) Use of multiplex PCR and PCR restriction enzyme analysis for detection and exploration of the variability in the free-living amoeba Naegleria in the environment. Appl Environ Microb 68(4):2061–2065
Booton GC, Schuster FL, Carmichael JR, Fuerst PA, Byers TJ (2003) Balamuthia mandrillaris: identification of clinical and environmental isolates using genus-specific PCR. J J Eukaryot Microbiol 50(6):508–510
Page FC (1988) A new key to freshwater and soil gymnamoebae. The fresh water biological association. The Ferry House, Ambleside
Liang SY, Ji DR, Hsia KT, Hung CC, Sheng WH, Hsu BM, Chen JS, Wu MH, Lai CH, Ji DD (2010) Isolation and identification of Acanthamoeba species related to amoebic encephalitis and non-pathogenic free-living amoeba species from the rice field. J Appl Microbiol 109:1422–1429
Sawyer TK (1989) Free-living pathogenic and non-pathogenic amoebae in Maryland soils. Appl Environ Microbiol 55:1074–1077
Todd CD, Reyes-Batlle M, Martín-Navarro CM, Dorta-Gorrín A, López-Arencibia A, Martínez-Carretero E, Piñero JE, Valladares B, Lindo JF, Lorenzo-Morales J (2015) Isolation and genotyping of Acanthamoeba strains from soil sources from Jamaica, West Indies. J Eukaryot Microbiol 62(3):416–421
Walochnik J, Scheikl U, Haller-Schoberb EM (2015) Twenty yearsof acanthamoeba diagnostics in Austria. J Eukaryot Microbiol 62(1):3–11
Barete S, Combes A, de Jonckheere JF, Datry A, Varnous S, Martinez V, García Ptacek S, Caumes E, Capron F, Francès C, Gibert C, Chosidow O (2007) Fatal disseminated acanthamoeba lenticulata acanthamebiasis in a heart transplant patient. Emerg Infect Dis 13(5):736–738
Ledee DR, Iovieno A, Miller D, Mandal N, Diaz M, Fell J, Fini ME, Alfonso EC (2009) Molecular identification of T4 and T5 genotypes in isolates from Acanthamoeba keratitis patients. J Clin Microbiol 47:1458–1462
Ledee DR, Miller D, Alfonso EC (2010) Detection of bacterial endosymbionts in clinical Acanthamoeba isolates. Ophthalmology 117(3):445–452.e443
Huang S-W, Hsu B-M (2011) Survey of Naegleria from Taiwan recreational waters using culture enrichment combined with PCR. Acta Trop 119(2–3):114–118
Jonckheere JFD (2006) Isolation and Molecular Identification of Vahlkampfiid Amoebae from an Island (Tenerife, Spain). Acta Protozoo 3(1):91–96
Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M (2003) Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol 41(7):3175–3180
Baquero RA, Reyes-Batlle M, Nicola GG, Martín-Navarro CM, Lopez-Arencibia A, Guillermo Esteban J, Valladares B, Martínez-Carretero E, Pinero JE, Lorenzo-Morales J (2014) Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health 108(4):206–211
Cabello-Vílchez AM, Reyes-Batlle M, Montalbán-Sandoval E, Martín-Navarro CM, López-Arencibia A, Elias-Letts R, Guerra H, Gotuzzo E, Martínez-Carretero E, Piñero JE (2014) The isolation of Balamuthia mandrillaris from environmental sources from Peru. Parasitol Res 113(7):2509–2513
Lares-Jiménez LF, Booton GC, Lares-Villa F, Velázquez-Contreras CA, Fuerst PA (2014) Genetic analysis among environmental strains of Balamuthia mandrillaris recovered from an artificial lagoon and from soil in Sonora, Mexico. Exp Parasitol 145:S57–S61
Yamanouchi K, Arima H, Sakamoto Y, Kanto K, Kasai K, Ito K, Inaba T (2018) First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitol Res 117(9):2895–2900
Funding
This study was funded by the Vice-Chancellor of Research Affairs, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No: 13660-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest
Ethical Statements
The Ethics Committee of Shahid Beheshti University of Medical Sciences in Iran approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pazoki, H., Niyyati, M., Javanmard, E. et al. Isolation and Phylogenetic Analysis of Free-Living Amoebae (Acanthamoeba, Naegleria, and Vermamoeba) in the Farmland Soils and Recreational Places in Iran. Acta Parasit. 65, 36–43 (2020). https://doi.org/10.2478/s11686-019-00126-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00126-9