Abstract
Introducction
Trichomonas vaginalis is a highly prevalent parasitic that causes the sexually transmitted disease trichomoniasis with some serious health complications. More understanding about genetic features of the parasite can be helpful in the study of the pathogenesis, epidemiology of the infection and drug susceptibility. For this end, we conducted analysis of a fragment (23 kDa) of the p60 of T. vaginalis gene.
Material and methods
The restriction fragment length polymorphism (RFLP) methods was used.
Result and discussion
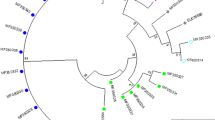
RFLP analysis showed the difference between T. vaginalis isolates from symptomatic and asymptomatic patients, suggesting a relation between the genetic identity of the isolates and their clinical manifestations.



Similar content being viewed by others
References
Petrin D, Delgaty K, Bhatt R, Garber G (1998) Clinical and microbiological aspects of ` + Trichomonas vaginalis. Clin Microbiol Rev 11:300–317
Hobbs MM, Seña AC (2013) Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect 89:434–438
Hitti J, Nugent R, Boutain D, Gardella C, Hillier SL, Eschenbach DA (2007) Racial disparity in risk of preterm birth associated with lower genital tract infection. Paediatr Perinat Epidemiol 21:330–337
Meites E, Gaydos CA, Hobbs MM, Kissinger P, Nyirjesy P, Schwebke JR, Secor WE, Sobel JD, Workowski KA (2015) A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis 15:S837–S848
Guenthner PC, Secor WE, Dezzutti CS (2005) Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun 73:4155–4160
McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, Ndinya-Achola J, Jaoko W, Baeten JM (2007) Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 195:698–702
Braz C, Piccoli A, Tasca T (2016) Trichomoniasis—are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microbial Cell 3(9):404–419
Viikki M, Pukkala E, Hakama M (2000) Gynecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol 39:71–75
Hide M, Marion E, Pomares C, Fisa R, Marty P, Bañuls AL (2013) Parasitic genotypes appear to differ in leishmaniasis patients compared with asymptomatic related carriers. Int J Parasitol 43:389–397
Puebla LJ, Núñez FA, Fernández YA, Fraga J, Rivero LR, Millán IA, Valdés LA, Silva IM (2014) Correlation of Giardia duodenalis assemblages with clinical and epidemiological data in Cuban children. Infect Genet Evol 23:7–12
Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de Peer Y, Miranda-Saavedra D, Barton GJ, Westrop GD, Müller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM Jr, Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM (2007) Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212
Alderete JF, Demĕs P, Gombosová A, Valent M, Yánoska A, Fabusová H, Kasmala L, Garza GE, Metcalfe EC (1987) Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun 55:1037–1041
Crucitti T, Abdellati S, Van Dyck E, Buve A (2008) Molecular typing of the actin gene of Trichomonas vaginalis isolates by PCR-restriction fragment length polymorphism. Clin Microbiol Infect 14:844–852
Hampl V, Vanacova S, Kulda J, Flegr J (2001) Concordance between genetic relatedness and phenotypic similarities of Trichomonas vaginalis strains. BMC Evol Biol 1:11. https://doi.org/10.1186/1471-2148-1-11
Rojas L, Fraga J, Sariego I (2004) Genetic variability between Trichomonas vaginalis isolates and correlation with clinical presentation. Infect Genet Evol 4:53–58
Ryu JS, Min DY, Shin MH, Cho YH (1998) Genetic variance of Trichomonas vaginalis isolates by Southern hybridization. Korean J Parasitol 36:207–211
Snipes LJ, Gamard PM, Narcisi EM, Beard CB, Lehmann T, Secor WE (2000) Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J Clin Microbiol 38:3004–3009
Stiles JK, Shah PH, Xue L, Meade JC, Lushbaugh WB, Cleary JD, Finley RW (2000) Molecular typing of Trichomonas vaginalis isolates by HSP70 restriction fragment length polymorphism. Am J Trop Med Hyg 62:441–445
Upcroft JA, Delgadillo-Correa MG, Dunne RL, Sturm AW, Johnson PJ, Upcroft P (2006) Genotyping Trichomonas vaginalis. Int J Parasitol 36:821–828
Vanacova S, Tachezy J, Kulda J, Flegr J (1997) Characterization of trichomonad species and strains by PCR fingerprinting. J Eukaryot Microbiol 44:545–552
Cornelius DC, Robinson DA, Muzny CA, Mena LA, Aanensen DM, Lushbaugh WB, Meade JC (2012) Genetic characterization of Trichomonas vaginalis Isolates by use of multilocus sequence typing. J Clin Microbiol 50:3293–3300
Conrad M, Zubacova Z, Dunn LA, Upcroft J, Sullivan SA, Tachezy J, Carlton JM (2011) Microsatellite polymorphism in the sexually transmitted human pathogen Trichomonas vaginalis indicates a genetically diverse parasite. Mol Biochem Parasitol 175:30–38
Meng M, Hui LL (2015) Genetic diversity of Trichomonas vaginalis clinical isolates from Henan province in central China. Pathog Glob Health 109:242–246
Hernández HM, Marcet R, Sarracent J (2014) Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite 21:54
Garber GE, Lemchuk-Favel LT (1994) Analysis of the extracellular proteases of Trichomonas vaginalis. Parasitol Res 80:361–365
Hernández H, Sariego I, Alvarez AB, Marcet R, Vancol E, Alvarez A, Figueredo M, Sarracent J (2011) Trichomonas vaginalis 62 kDa proteinase as a possible virulence factor. Parasitol Res 108:241–245
Hernández H, Sariego I, Garber G, Delgado R, López O, Sarracent J (2004) Monoclonal antibodies against a 62 kDa proteinase of Trichomonas vaginalis decrease parasite cytoadherence to epithelial cells and confer protection in mice. Parasite Immunol 26:119–125
Garber GE, Lemchuk-Favel LT, Meysick KC, Dimock KA (1993) Trichomonas vaginalis cDNA with partial protein sequence homology with a Plasmodium falciparum excreted protein ABRA. Appl Parasitol 34:245–249
Altschul SF, Madden TL, Schiffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLST and PSI-BLAST a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Tamura K, Dudley J, Nei M, Kumar S (1997) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Rojas L, Izquierdo A, Sarría CA, Sarracent J, Sánchez L (2003) Comportamiento de la Trichomonosis vaginal en un grupo de adolescente. Revista Cubana de Medina Tropical 55:179–184
Diamond LS, Harlow OR, Cunnick CC (1978) A new medium for axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72:431–434
Maniatis T, Fritsch EF, Sambrook J (1990) Molecular cloning: a laboratory manual. Cold Spring Harber Laboratory Press, Cold Spring Harber
Sneath PHA (1957) The application of computers to taxonomy. J Genet Microbiol 17:201–226
Sneath PHA, Sokal RR (1973) Numerical taxonomy: the principles and practice of numerical classification. W. H. Freeman, San Francisco, p 573p
Pavlícek A, Hradá S, Flegr J (1999) Free-Tree–freeware program for construction of phylogenetic trees on the basis of distance data and bootstrap/jackknife analysis of the tree robustness. Application in the RAPD analysis of genus Frenkelia. Folia Biol 45:97–99
Meade JC, Carlton JM (2013) Genetic diversity in Trichomonas vaginalis. Sex Transm Infect 89:444–448
Alka D, Sarin BC, Mittar D, Sehajpal PK (2003) Optimization of 38 kDa based PCR assay for detection of Mycobacterium tuberculosis from clinical samples. Indian J Tuberc 50:209
Khosravinia H, Ramescha KP (2007) Influence of EDTA and magnesium on DNA extraction from blood samples and specificity of polymerase chain reaction. Afr J Biotech 6:184–187
Grunenwald H (2006) Methods in molecular biology. In: Bartlett JMS, Stirling D (eds) The PCR revolution. Humana Press Inc, Totowa, p 90
Roche (2006) PCR applications manual, p 34
Init I, Mak JW, Lokman Hakim S, Yong HS (1999) Strain differences in Blastocystis isolates as detected by a single set of polymerase chain reaction primers. Parasitol Res 85:131–134
Init I, Foead AI, Fong MY, Yamazaki H, Rohela M, Yong HS, Mak JW (2007) Restriction enzyme digestion analysis of PCR-amplified DNA of Blastocystis hominis isolates. Southeast Asian J Trop Med Public Health 38:991–997
Praneet K, Gupta I, Sehgal R, Malla N (2004) Trichomonas vaginalis: random amplified polymorphic DNA analysis of isolates from symptomatic and asymptomatic women in India. Parasitol Int 53:255–262
Fraga J, Rojas L, Sariego I, Fernández-Calienes A (2011) Double-stranded RNA viral infection of Trichomonas vaginalis and correlation with genetic polymorphism of isolates. Exp Parasitol 127:593–599
Matini M, Rezaie S, Mohebali M, Maghsood AH, Rabiee S, Fallah M, Rezaeian M (2014) Genetic identification of Trichomonas vaginalis by using the actin gene and molecular based methods. Iran J Parasitol 9:329–335
Meade JC, de Mestral J, Stiles JK, Secor WE, Finley RW, Cleary JD, Lushbaugh WB (2009) Genetic diversity of Trichomonas vaginalis clinical isolates determined by EcoRI restriction fragment length polymorphism of heat-shock protein 70 genes. Am J Trop Med Hyg 80:245–251
Simões-Barbosa A, Lobo TT, Xavier J, Carvalho SE, Leornadecz E (2005) Trichomonas vaginalis: intrastrain polymorphisms within the ribosomal intergenic spacer do not correlate with clinical presentation. Exp Parasitol 110:108–113
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández, H., Fraga, J., Marcet, R. et al. Genetic Diversity of Trichomonas Vaginalis Clinical Isolates According to Restriction Fragment Length Polymorphism Analysis of the 60-kDa Proteinase Gene. Acta Parasit. 64, 300–307 (2019). https://doi.org/10.2478/s11686-019-00065-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00065-5