Abstract
Fabry disease, an X-linked glycosphingolipid storage disorder, is caused by the deficient activity of α-galactosidase A (α-Gal A). This results in the lysosomal accumulation in various cell types of its glycolipid substrates, including globotriaosylceramide (GL-3) and lysoglobotriaosylceramide (globotriaosyl lysosphingolipid, lyso-GL-3), leading to kidney, heart, and cerebrovascular disease. To complement and potentially augment the current standard of care, biweekly infusions of recombinant α-Gal A, the merits of substrate reduction therapy (SRT) by selectively inhibiting glucosylceramide synthase (GCS) were examined. Here, we report the development of a novel, orally available GCS inhibitor (Genz-682452) with pharmacological and safety profiles that have potential for treating Fabry disease. Treating Fabry mice with Genz-682452 resulted in reduced tissue levels of GL-3 and lyso-GL-3 and a delayed loss of the thermal nociceptive response. Greatest improvements were realized when the therapeutic intervention was administered to younger mice before they developed overt pathology. Importantly, as the pharmacologic profiles of α-Gal A and Genz-682452 are different, treating animals with both drugs conferred the greatest efficacy. For example, because Genz-682452, but not α-Gal A, can traverse the blood-brain barrier, levels of accumulated glycosphingolipids were reduced in the brain of Genz-682452-treated but not α-Gal A-treated mice. These results suggest that combining substrate reduction and enzyme replacement may confer both complementary and additive therapeutic benefits in Fabry disease.
Similar content being viewed by others
Introduction
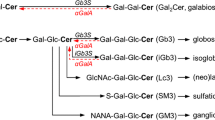
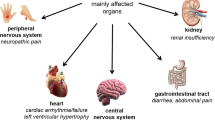
Fabry disease is an X-linked inherited metabolic disorder caused by the deficient activity of the lysosomal hydrolase α-galactosidase A (α-Gal A) (1). Progressive lysosomal accumulation of globotriaosylceramide (GL-3) and related glycolipid substrates leads to a number of clinical manifestations that define the two major Fabry disease phenotypes. The early-onset, severe “classic” Type 1 phenotype has little (<1%) or no functional α-Gal A activity, marked microvascular endothelial substrate accumulation, childhood/adolescent onset of angiokeratoma, acroparesthesias, hypohidrosis and gastrointestinal symptoms, and a characteristic keratopathy. With age, the Type 1 phenotype progresses to hypertrophic cardiomyopathy, renal failure, and/or cerebrovascular disease, and early demise. The “later-onset” Type 2 phenotype has residual α-Gal A activity (>1%) and no microvascular endothelial substrate accumulation or early Type 1 manifestations, but it progresses to renal and cardiac disease, typically during or after the third decade of life (1).
The current standard of care for Fabry disease, whether Type 1 classical or Type 2 later onset, is enzyme replacement therapy (ERT). Biweekly infusions of recombinant human α-Gal A (rh α-Gal A) effectively reduce the GL-3 and lyso-GL-3 in a variety of cells, reversing substrate accumulation and disease manifestations (2–6). ERT also reduces substrate levels in other affected cells such as renal peritubular (interstitial) cells, the capillary endothelia of heart, liver and skin, as well as from plasma and urinary sediments (7–9). Recent reports substantiate previous observations that earlier treatment results in the best outcomes (10). It should be noted that the rate and extent of clearance varies, with some cell types in the kidney (podocytes and distal tubular epithelial cells) and heart (cardiomyocytes) being more refractory to treatment (9).
Although the pivotal clinical trials with ERT intimated a reduction in pain, longer-term studies in adults on ERT have been met with mixed results because treatment initiation typically began in the fourth to fifth decades of life (7,11–14). On the basis of the clinical experience with ERT, it is evident that Fabry patients may benefit from earlier ERT as well as from new adjunctive therapies that can more effectively reduce systemic substrate accumulation.
Substrate reduction therapy (SRT) is gaining interest as an alternate approach to reduce levels of the metabolites that accumulate in Fabry disease by decreasing the synthesis of relevant precursor glycosphingolipids. This concept has already been shown to be effective in the management of Gaucher disease, another glycosphingolipidosis (15,16). For both Gaucher disease and Fabry disease, substrate reduction may be realized by inhibiting glucosylceramide synthase (GCS), the enzyme that catalyzes the first step in the synthesis of glucosphingolipids. As an orally available antagonist of GCS, it acts in a mechanistically distinct manner from the enzyme, such that this therapeutic concept may confer complementary and potentially additive benefits to ERT.
We previously reported on the merits of SRT either as a standalone monotherapy or as an adjunctive therapy to ERT using a GCS inhibitor, Genz-112638 (eliglustat), in both Gaucher and Fabry mice (17,18). Here, we describe studies with Genz-682452, a novel, selective and potent GCS inhibitor with central nervous system (CNS) access (19) that exhibits a pharmacokinetic and safety profile appropriate for Fabry disease. We confirmed that SRT with Genz-682452 can provide an effective approach to lowering the pathologic accumulation of the major glycolipid substrates in a mouse model of Fabry disease. Furthermore, as the pharmacodynamic profiles and mechanistic bases of the two therapeutic modalities are distinct, evidence of therapeutic complementation and in some tissues indications of an additive effect were observed. As such, the availability of Genz-682452 represents an adjuvant therapy that may improve the quality of care for patients with Fabry disease.
Materials and Methods
Animal Procedures
Procedures involving mice were reviewed and approved by Genzyme Corporation’s Institutional Animal Care and Use Committee following guidelines established by the Association for Assessment of Accreditation of Laboratory Animal Care (AAALAC). Wild-type 129SvEv mice were obtained from Taconic Laboratories (Germantown, NY). Fabry mice, both affected males and homozygous females (α-galactosidase A knockout mice), were bred at Charles River Labs (Bedford, MA) (20).
Urine was collected from mice housed individually in metabolic cages and they were kept for 24 h with unrestricted access to food and water. Blood samples were collected from the orbital venous plexus under anesthesia (2% to 3% isoflurane) into EDTA microhematocrit capillary tubes. Animals were killed by carbon dioxide inhalation, and their tissues were harvested immediately and then snap frozen on dry ice or drop fixed in 10% neutral buffered formalin. Spinal columns were taken whole and fixed in 10% neutral buffered formalin before processing for histological analysis.
Treatment Regimens
All studies to evaluate the combined effects of rh α-Gal A and Genz-682452 were performed in male Fabry mice. Prior to treatment, mice received a tail vein injection of a recombinant adeno-associated virus (AAV) vector (AAV2/8 agalD170Mut) expressing an inactive form (created by a point mutation in amino acid 170 to code alanine in place of aspartic acid) of human α-Gal A to induce immune tolerance to the enzyme. One-month-old mice were administered 1 × 1011 DNase-resistant particles (drp) of AAV2/8 agalD170Mut and then allowed to mature to 3 months of age prior to starting the drug treatments. Male mice were used for this study as the efficiency of AAV-mediated hepatic transduction is significantly greater in male than in female mice (21,22).
All drug treatments were initiated in 3-month-old mice. Mice received Genz-682452 as a component of their standard pelleted rodent diet throughout the studies; the drug was formulated at 0.03% w/w in LabDiet 5053 (TestDiet, Richmond, IN). This formulation provided ∼60 mg of Genz-682452/kg per day for a 25-g mouse eating 5 g of food per day. This dose was selected based on earlier pilot tolerability and efficacy studies (data not shown). Mice in the appropriate treatment groups received 1 mg/kg rh α-Gal A via tail vein injections every other month at 3, 5, 7, 9 and 11 months of age. The ERT dose was selected on the basis of previous studies (18,23) and unpublished findings.
A study to examine the effects of longer-term SRT only was performed using the female littermates of the male mice used for the ERT and SRT study described above. In this study the female mice received SRT with the same formulation of Genz-682452 in their diets as for the male combination therapy above. Cohorts of mice began SRT at 3, 8, and 12 months of age and were treated until 17 months old.
Measurement of Peripheral Sensory Function Using the Hot-Plate Test
A test of nociceptive response to a heat stimulus was performed monthly as described previously (21). Mice were placed on a 55°C surface (analgesia meter, Columbus Instruments, Columbus, OH) and the time taken to respond, as illustrated by a characteristic hind paw shake, was recorded as the latency. If no response was evident by 60 s, the mouse was removed to prevent injury.
Antibodies to α-Galactosidase A
Plasma samples were assayed using a whole immunoglobulin G (IgG) antibody enzyme-linked immunosorbant assay (ELISA) assay. Briefly, clear 96-well ELISA plates were coated with 1 µg/mL rh α-Gal A and incubated overnight at 4°C. Plates were blocked with 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for at least 1 h at 37°C, after which the samples were serially diluted (range 1:200–1:6400) and incubated for an additional hour at 37°C. Antibodies binding rh α-Gal A were detected using a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10,000) (Jackson ImmunoResearch Labs, West Grove, PA). Samples were incubated for 1 h at 37°C and then visualized using the BioFX TMB/Stop solution (SurModics, Inc., Eden Prairie, MN). Plates were read on a Molecular Devices M3 plate reader using the SoftMax 5.4.1 software (Molecular Devices, Sunnyvale, CA, USA) at an absorbance of 450 nm with A540 subtracted for background. The anti-α-Gal A titers were defined as the reciprocal of the lowest dilution giving an optical density (OD) of ≥0.1.
Sphingolipid Analysis
Quantitative analysis of sphingolipids was performed by liquid chromatography and tandem mass spectrometry (LC/MS/MS). Briefly, tissue was homogenized in 10 times its volume of water (w/v) and 10 µL of homogenate was extracted with 1 mL of an organic solvent mixture. For the extraction of GL-3 and lyso-GL-3, the solvents consisted of acetonitrile, methanol, acetic acid, 75/25/1 (v/v/v), and 5 mmol/L ammonium acetate. For the extraction of GL-1, galactosylceramide, GL-2 and di-galactosylceramide, the solvents consisted of acetonitrile, methanol, acetic acid, 90/10/1 (v/v/v), and 5 mmol/L ammonium acetate. The samples were placed on a VX-2500 tube vortexer (VWR International, Radnor, PA) for 5 min and then centrifuged for 4 min at 10,000g. The resulting supernatant was transferred into high-performance liquid chromatography (HPLC) vials for analysis. For GL-3 and lyso-GL-3 analyses, an Acquity BEH HILIC column (2.1 × 100 mm, 1.7-µm particle [“BEH HILIC” means “ethylene bridged hybrid hydrophilic interaction liquid chromatography]; Waters Corp., Milford, MA) was used to separate the sphingolipids that were then analyzed by triple quadrupole tandem mass spectrometry (API 4000; AB Sciex, Framingham, MA) using the multiple reaction monitoring (MRM) mode. GL-2 and digalactosylceramide were analyzed similarly to GL-3 but with a modified liquid chromatography (LC) gradient. Two Atlantis HILIC columns in tandem (2.1 × 150 mm, 3-µm particle; Waters Corp., Milford, MA) were used to separate GL-1 and galactosylceramide prior to detection by tandem mass spectrometry (API 4000). Sphingolipid standards were obtained from Matreya, LLC (Pleasant Gap, PA).
Histopathology
At select time points throughout the study, mice were euthanized by carbon dioxide asphyxiation. Whole spinal columns were fixed in 10% neutral buffered formalin and then decalcified using buffered formic acid for 5 to 7 d. Decalcified spinal columns containing the spinal cord and dorsal root ganglia (DRG) were processed as previously described and then embedded in paraffin. Cross sections (5–6 µm) were then stained with hematoxylin and eosin (H&E) (18). A board-certified veterinary pathologist blinded to the study design examined multiple sections of the spinal column, including the spinal cord and DRG, microscopically (Nikon Eclipse 80i microscope and Nikon DS-Ri digital camera, Nikon Instruments Inc., Melville, NY). For each section, the numbers of normal and enlarged/vacuolated DRG cells were manually counted in a representative microscopic field at 400× magnification. For quantitative assessment, the percent of vacuolated DRG cells in each section was calculated by dividing the number of vacuolated DRG cells by the total number of DRG cells (18).
Results
Genz-682452 Specifically Lowers Glucosphingolipid Levels in the Kidneys of Fabry Disease and Wild-Type Mice
To examine the activity and specificity of the novel inhibitor Genz-682452, we measured the levels of ceramide-based glycolipids in the kidneys of Fabry and wild-type mice administered Genz-682452 or vehicle over a period of 9 months. Animals were treated with Genz-682452 or vehicle starting at 3 months of age. As expected, analysis of the glucosphingolipids GL-1 and GL-2 in the kidney of untreated control Fabry mice at the end of the study (when the animals were 1 year old) showed they were similar to those in wild-type mice (Figure 1). However, kidneys of both Fabry and wild-type mice treated with Genz-682452 showed reductions of GL-1 (∼85%) and GL-2 (∼60%) (Figure 1A, B). The extent of the reduction was similar in male and female mice.
Effect of Genz-682452 on kidney glycosphingolipid levels. GL-1 (A), GL-2 (B), galactosylceramide (GalCer) (C) and di-galactosylceramide (GalGalCer) (D) levels in kidneys of 12-month-old mice. WT-UNT, untreated wild-type mice; Fabry-UNT, untreated Fabry mice; Fabry-SRT, substrate reduction therapy in Fabry mice; WT-SRT, substrate reduction therapy in wild-type mice. Animals were administered Genz-682452 through their diet for 9 months. Data for male mice (n = 9–12 mice) and for female mice (n = 4 mice) are presented as mean ± SD. Male and female mice were analyzed separately by one-way analysis of variance (ANOVA) p < 0.0001. Dunnett posttest was performed with Fabry-UNT as the reference. (A) Male: Fabry-UNT and WT-UNT ns (not significant), Fabry-SRT p < 0.0001; female: Fabry-UN and WT-UNT ns, Fabry-SRT p < 0.0001, WT-SRT P < 0.0001. (B) Male: Fabry-UNT and WT-UNT p < 0.0001, Fabry-SRT p < 0.0001; female: Fabry-UNT and WT-UNT p < 0.05, Fabry-SRT p < 0.01, WT-SRT p < 0.01. (C) Male: Fabry-UNT and WT-UNT p < 0.05, Fabry-SRT p < 0.0001; female: Fabry-UNT and WT-UNT ns, Fabry-SRT ns, WT-SRT p < 0.05. (D) Male: Fabry-UNT and WT-UNT p < 0.001, Fabry-SRT p < 0.001; female: Fabry-UNT, WT-UNT, Fabry-SRT and WT-SRT ns.
To examine the specificity of the inhibitor, we also analyzed kidney galactosylceramide (GalCer) and digalactosylceramide (GalGalCer) levels (Figure 1C, D). GalCer levels were elevated ∼5-fold in the kidneys of wild-type and Fabry male relative to female mice, an observation that has been reported for many strains of mice (24,25). GalCer and GalGalCer are glycolipids that are reportedly elevated in Fabry patients but whose metabolism should not be affected by antagonism of GCS. It should be noted that GalGalCer is also a substrate for α-Gal A, though its pathological relevance is not known (1,26,27). Indeed, in male Fabry mice, we observed higher levels of GalGalCer than in wild-type controls (Figure 1D). As expected, treatment with Genz-682452 did not reduce the levels of either GalCer or GalGalCer asserting that inhibition of GCS primarily affected only those glycolipids that were downstream of this synthetic step. In fact, a slight elevation of these galactosphingolipids was noted in the treated male Fabry mice, which may have been caused by the greater availability of ceramide due to inhibition of glucosphingolipid synthesis by Genz-682452.
Combination Therapy with Genz-682452 and α-Galactosidase A Is More Efficacious than Either Therapy Alone at Reducing Glycosphingolipid Levels in Fabry Mice
The ability of Genz-682452 to lower the levels of GL-3 and lyso-GL-3, the major glycosphingolipids that accumulate in Fabry disease, was also examined. In addition, as the mechanism of action of SRT with Genz-682452 is different from enzyme therapy with α-Gal A, we determined if the combined use of both therapies was additive. For the enzyme alone and combination therapy studies, Fabry mice were first immunotolerized to the recombinant human enzyme by treating the animals with a recombinant AAV vector encoding an inactive, but stable mutant form of α-Gal A (point mutation in amino acid 170). We and others have previously shown that AAV-mediated hepatic-restricted expression of proteins resulted in the induction of immune tolerance to the expressed protein (22,28–32). Administration of rh α-Gal A into AAV2/8 α-galD170Mut-treated Fabry mice did not elicit antibodies to the enzyme (data not shown). Thus, rh α-Gal A could be administered bimonthly and as previous studies have shown this regimen only partially reduces GL-3, thereby allowing for the potential to measure greater efficacy when combined with SRT.
As reported previously, the levels of GL-3 and lyso-GL-3 in the visceral tissues (kidney, spleen, liver, heart and lung) of Fabry mice increased progressively with age (18). Mice treated for 9 months with either recombinant enzyme or Genz-682452 alone showed significantly lower levels of both GL-3 and lyso-GL-3 in all visceral tissues assayed (Figures 2 and 3). However, the greatest reduction was observed in mice administered both rh α-Gal A and Genz-682452. The combination therapy resulted in >90% reduction of GL-3 and 75%–90% reduction of lyso-GL-3 after 9 months of treatment (Table 1). In kidney and liver, the levels normalized to those of wild-type mice.
Relative tissue levels of GL-3 in Fabry mice. GL-3 levels are normalized to control age-matched untreated Fabry mice. The baseline values in 3-month-old mice were set at 100%. Relative GL-3 levels are shown for kidney, spleen, liver, heart, lung and brain (as indicated) at 3, 6, 9 and 12 months of age. E + S, combined ERT and SRT. Each data point represents the mean of 4 mice except those measured at 12 months, which represent the mean of 9–11 mice ± SD.
Relative tissue levels of lyso-GL-3 in Fabry mice. Lyso-GL-3 levels are normalized to control age-matched untreated Fabry mice. The baseline values in 3-month-old mice were set at 100%. Relative GL-3 levels are shown for kidney, spleen, liver, heart, lung and brain (as indicated) at 3, 6, 9 and 12 months of age. E + S, combined ERT and SRT. Each data point represents the mean of 4 mice except those measured at 12 months, which represent the mean of 9–11 mice ± SD.
Brains of Fabry mice exhibited modestly elevated levels of the accumulated glycosphingolipids compared with age-matched controls (Figures 2 and 3). As expected, treatment with rh α-Gal A alone did not alter the brain levels of GL-3 and lyso-GL-3 because intravenously infused enzyme is unable to traverse the blood-brain barrier. In contrast, mice administered Genz-682452, which has CNS exposure, showed an approximately 40% lower level of GL-3 (Figure 2 and Table 1) and 25% lower level of lyso-GL-3 (Figure 3) in the brain. As expected, Fabry mice treated with a combination of SRT and ERT showed a similar reduction in both lipids in the brain as Fabry mice treated with SRT alone (Table 1). Hence, because Genz-682452 and rh α-Gal A exhibit different pharmacodynamic profiles, their combined use provided complementary and, in some tissues, additive benefits.
Measurement of plasma levels of GL-3 and lyso-GL-3 showed a similar pattern of reduction to that noted in the visceral organs. Lower levels of the glycosphingolipids were observed with either therapy alone, with the greatest reduction attained using the combination therapy (Figure 4). As such, measurements of plasma lipid levels may represent a surrogate biomarker for efficacy in the visceral tissues. Interestingly, elevated GL-3 levels in urine were not altered in Fabry mice treated with rh α-Gal A alone, whereas GL-3 levels were corrected to near normal in mice administered Genz-682452 alone (Figure 4). This observation reinforces the merit of combination therapy with rh α-Gal A and Genz-682425 to confer complementary benefits. If these benefits are realized in Fabry patients, Genz-682452 could address aspects of the disease that are less well met by currently available enzyme therapies.
Relative levels of the substrates GL-3 and lyso-GL-3 in the plasma and urine from Fabry mice. The effect of the different treatments (ERT, SRT and E + S) on substrate levels (GL-3 or lyso-GL-3) in the plasma and urine of are shown. Data were normalized to age-matched untreated Fabry mice at 4, 8, 10 and 12 months of age. E + S, combined ERT and SRT. Each data point represents the mean of 8–10 mice ± SD.
SRT with Genz-682452 Reduces Accumulation of Substrates in the Small Intestine
Delayed gastric emptying, diarrhea, and gastrointestinal cramping and pain are commonly reported symptoms in Fabry patients with the Type 1 classic phenotype (33). Although corresponding symptoms have not been reported in Fabry mice, notable pathological inclusions present in the smooth muscle cells and myenteric plexus of the animals’ small intestines stain positively for CD77, a marker of GL-3 (34). Indeed, quantitation confirmed that GL-3 levels in the small intestine of Fabry mice are as highly elevated as in other visceral tissues (Figure 5). In a separate study, female Fabry mice were treated with Genz-682452 at 3, 8, or 12 months of age and their intestinal GL-3 levels were evaluated at 17 months of age (corresponding to treatment periods of 14, 9 and 5 months, respectively). As shown in Figure 5, Genz-682452-treated mice had significantly lower GL-3 levels in small intestine than untreated controls if SRT was initiated by 8 months of age. The animals treated for the longest period showed the greatest reduction in substrate accumulation.
Effect of SRT on the levels of GL-3 in the small intestine of Fabry mice. Fabry mice (female) were treated at 3 (SRT3), 8 (SRT8) or 12 (SRT12) months of age with Genz-682452 (60 mg/kg per day in pelleted diet) until 17 months old and the levels of GL-3 in the small intestines were measured. GL-3 levels in untreated Fabry mice (UNT) and wild-type mice (WT-UNT) are also shown. Each bar represents the mean of 4 mice ± SD. Statistics were performed using one-way analysis of variance (ANOVA) p < 0.0001, Tukey multiple comparison. SRT3 and SRT8 are significantly different from UNT p < 0.0001, SRT12 versus UNT ns (not significant), SRT3 versus SRT8 p < 0.05, SRT8 versus SRT12 p < 0.001, SRT3 versus SRT12 p < 0.0001.
SRT with Genz-682452 Is More Effective at Reducing Tissue GL-3 Levels in Younger than in Older Fabry Mice
To examine the relative merits of SRT at addressing the tissue accumulation of GL-3 in young and older Fabry mice (the latter with a higher baseline level of substrate), cohorts of animals were treated with Genz-682452 starting at 3 and 12 months of age. After 5 months of treatment (when the mice were 8 and 17 months old, respectively), the levels of GL-3 in the kidney, heart, lung, spleen and brain were measured and compared with those from age-matched untreated animals. As shown in Figure 6, Fabry mice administered Genz-682452 showed lower tissue levels of GL-3 compared with their untreated age-matched controls. However, mice starting treatment at a younger age had lower tissue levels of GL-3 than those that started treatment when older (Figure 6). For example, in the kidney of Fabry mice that received drug starting at 3 months old, a 90% reduction in the substrate level was attained compared with only ∼25% in those mice initially treated at 12 months of age. Correspondingly, lower levels of GL-3 were also noted in other visceral organs of Fabry mice that received drug at 3 months old, albeit to a lesser extent. Interestingly, although treatment of younger Fabry mice resulted in an ∼60% lower level of GL-3 in the brain, treatment of the older cohort did not alter the GL-3 level (Figure 6), arguing for the importance of early intervention.
Effect of initiating SRT in 3- and 8-month-old Fabry mice on their GL-3 levels. GL-3 levels are expressed as a percentage of the levels in untreated age-matched Fabry controls. “Fabry 8 months” represents animals treated by SRT starting at 3 months of age and with GL-3 levels analyzed at 8 months of age. “Fabry 17 months” represents animals treated by SRT starting at 12 months of age and with GL-3 levels analyzed at 17 months of age. The data reflect the percent reduction of GL-3 in the indicated tissues following 5 months of substrate reduction therapy. Each bar represents the mean of ≥4 mice.
SRT of Fabry Mice with Genz-682452 Delays the Onset of a Deficit in Their Thermosensory Responsiveness
To assess whether the different therapies also impacted the peripheral neuropathy noted in patients with classic Type 1 Fabry disease, mice were subjected to a test that measured their thermal nociceptive response. The animals were placed on a 55°C hotplate and their latency to respond to the heat was recorded. Male Fabry mice were treated for 9 months starting at 3 months of age with Genz-682452 or rh α-Gal A alone, or with a combination of the two drugs. In contrast to wild-type mice that showed a response time of 9–11 s, untreated Fabry mice exhibited a delayed latency to respond from 3 months of age that increased progressively with age (Figure 7A). As reported previously, Fabry mice treated by enzyme alone showed profile similar to that of untreated animals (18). However, animals treated with Genz-682452 alone or in combination with rh α-Gal A showed improved response times (starting at ∼4 months posttreatment) relative to the untreated mice. The latency to respond in the Genz-682452-treated Fabry mice at the end of the study (9 months posttreatment) was still greater than that in wildtype controls, suggesting that SRT primarily acts to abate the rate and extent of GL-3 accumulation as opposed to clearing the substrate from the affected cells (Figure 7A).
Thermal nociceptive response of male Fabry mice. Fabry mice were treated starting at 3 months of age with ERT (enzyme-replacement therapy), SRT (substrate-reduction therapy), or E + S (combination of ERT and SRT), and the effects of treatment were compared with untreated control Fabry (Fabry-UNT) or wild-type (WT-UNT) mice. (A) Thermal nociceptive responses (latency) were evaluated monthly at 3 months of age for 8 months. Each time point represents the mean of 10 mice ± S.D. Statistics were performed using two-way analysis of variance (ANOVA), p < 0.0001, Tukey multiple comparison of the final time point to either Fabry-UNT, **p < 0.01 or Fabry-ERT, *p < 0.05, ****p < 0.0001. (B) The percentage of mice that failed to respond to the 55°C hotplate within 60 s at the final time point (11 months old).
The thermal nociception response was also analyzed by counting the number of mice that reached the maximum latency period permitted (60 s) when placed on the hotplate at the end of the study (Figure 7B). Most of the untreated (∼90%) and all of the enzyme alone-treated animals failed to respond within 60 s when tested at the end of the study period. In contrast, 30% of the animals administered Genz-682452 alone and 10% of those treated with the combination of drugs exhibited this characteristic (Figure 7B). On the basis of this analysis, we surmised that treatment with Genz-682452 is more effective than rh α-Gal A alone at addressing this abnormality and that a combination of the therapies is modestly more effective.
Combination Therapy with rh α-Gal A and Genz-682452 Produces the Greatest Reduction in Cellular Vacuolization in the Dorsal Root Ganglia
To probe the basis for the differential thermal nociception response noted above, we performed hematoxylin and eosin (H&E) staining of tissue sections containing DRG. As reported previously, sections from 3-month-old untreated Fabry mice exhibit evidence of vacuolated cells in the efferent nerve bodies that stain positively for CD77 (34). H&E staining of sections from untreated Fabry mice at the end of the 9-month study period showed evidence of more extensive vacuolization (Figure 8A) (34). Mice subjected to rh α-Gal A, Genz-682452 or a combination of both drugs showed reduced vacuolization (Figure 8A). Quantitating the number of vacuolated DRG cells confirmed that all treatment groups had statistically significantly lower percentages of vacuolized cells compared with untreated Fabry mice (Figure 8B). Consistent with the results of the hot plate assay (Figure 7), a combination of ERT and SRT appeared to be most effective at preventing the development of vacuolized DRG cells and by extension, the loss of responsiveness to heat.
Effect of treatment on the extent of neuronal vacuolization in the DRG of Fabry mice. (A) Fabry mice (male) were treated for 9 months starting at 3 months old with ERT, SRT, or E + S (combination of ERT and SRT) and the effects of treatment were compared with untreated Fabry (Fabry-UNT) or wild-type (WT-UNT) mice. At the end of the study, the spinal columns were fixed and processed for H&E staining. In Fabry mice, vacuoles (arrows) were evident in the efferent nerve bodies in the DRG. (B) Quantitation of the percentage of DRG cells containing vacuoles indicated a therapeutic benefit from the treatments. Ctrl indicates the mean number of vacuolated DRG neurons in 3-month-old untreated Fabry mice. All treatment groups had significantly fewer vacuolated cells than the age-matched untreated Fabry mice (UNT). Statistics were performed using one-way analysis of variance (ANOVA), p < 0.0001; Dunnett posttest with UNT as the reference UNT versus ERT, p < 0.01, UNT versus SRT and E + S, p < 0.0001. (C) In a separate study with female Fabry mice, the percentages of DRG cells containing vacuoles were quantitated at 3, 8, 12 and 17 months of age. The mice were treated from 3 (SRT3), 8 (SRT8) or 12 (SRT12) months of age with Genz-682452 (60 mg/kg per day in pelleted diet) until 17 months old; arrows indicate timing of initiation of treatment for each group. The effects of treatment were compared with untreated control Fabry mice (UNT). At the final time point (17 months old), all treatment groups had a significantly lower percentage of vacuolated cells in their DRG compared with age-matched untreated controls (UNT). Mice treated at the youngest age (and for the longest period) showed the most significant reduction. Statistics were performed using one-way ANOVA p < 0.0001 with Dunnett multiple comparison posttest using UNT as the reference group, UNT versus SRT3, p < 0.0001, UNT versus SRT8 and SRT12 p < 0.05.
In a separate study, a cohort of homozygous female Fabry mice were administered Genz-682452 for varying periods of time and the effect of this intervention on DRG was examined. Untreated animals showed increasing vacuolization in their DRG cells with age (Figure 8C). When these animals were administered Genz-682452 starting at 3, 8 or 12 months of age, progression of the pathology in the DRG was halted. This was illustrated by the observation that the extent of vacuolization at 17 months of age was unchanged when compared with that measured at the start of their therapy (indicated by arrows). All treatment groups showed significantly lower extents of vacuolization in their DRG cells compared with the age-matched untreated Fabry mice at 17 months old. Hence, Fabry mice that were treated earlier and longer had the greatest pathologic benefit.
Discussion
Enzyme replacement therapy has been shown to be highly effective in correcting the disease manifestations of several lysosomal storage disorders including type 1 Gaucher, Pompe, mucopolysaccharidosis I (MPS I) and Fabry disease (16). However, not all disease manifestations are corrected by ERT, prompting the identification of potential adjunctive therapies. For example, although the lipids that accumulate in endothelial cells of Fabry disease are efficiently cleared by rh α-Gal A, other cell types such as the glomerular podocytes and cardiomyocytes are reportedly less responsive to enzyme therapy (9,35,36) unless treatment starts early (10). The basis for the differential responses is in part related to the biodistribution of the administered enzyme (23) and the need for early intervention. In this regard, an adjunctive therapy that exhibits a different pharmacodynamic profile to the enzyme could complement ERT and may provide an additive benefit for these cell types, especially when treatment is initiated in adult male patients.
Encouraged by the success of SRT for Gaucher disease (15,16), we investigated whether this therapeutic concept could also be applicable for treating Fabry disease. We focused on the same drug target as for Gaucher disease (that is, glucosyl-ceramide synthase), as antagonism of this enzyme with eliglustat (Genz-112638) has been shown to be safe and effective in human studies (37,38). Eliglustat was recently approved in the United States and the European Union as a firstline oral therapy in adult patients with Gaucher disease type 1 who are poor, intermediate, or extensive CYP2D6 metabolizers. Importantly, inhibition of GCS is also anticipated to lower GL-3 synthesis, the substrate that accumulates in Fabry disease, because glucosylceramide is a precursor of this glycolipid. Several lines of evidence support the notion that SRT may be effective for Fabry disease. We recently reported that treatment of induced pluripotent stem cells (iPSC)-derived cardiomyocytes from a Fabry patient with Genz-682452 prevented the accumulation of GL-3 (36). We also previously demonstrated the feasibility of this concept in Fabry mice by using an earlier generation inhibitor of GCS, Genz-112638 (18). Through systematic efforts in medicinal chemistry, a new inhibitor of GCS (Genz-682452) with the desired safety profile and characteristics for possible use in patients was developed and its effectiveness is documented in this report.
Treating Fabry mice with Genz-682452 demonstrated that the GCS inhibitor lowered GL-3 and lyso-GL-3 levels in several tissue compartments. The extent of the glycolipid decreases attained in most of the visceral tissues was even greater than that reported previously with Genz-112638 (18), reflecting the higher potency and improved pharmacokinetics of Genz-682452. Moreover, whereas Genz-112638 only reduced the rate of accumulation of the substrates, Genz-682452 produced a net lowering of these storage products in Fabry mice. Finally, as noted previously, mice treated with a combination of enzyme and Genz-682452 had the greatest reduction in GL-3 and lyso-GL-3. Treatment with Genz-682452 was well tolerated as both wild-type and Fabry mice treated with the drug for 14 months had no overt complications. Together, these studies support the utility of Genz-682452 for SRT in Fabry disease.
Here, we also extended the analysis to include the impact of SRT on the gastrointestinal tract, because Fabry patients report gastrointestinal disturbances such as postprandial pain and cramping. We recently reported that the Fabry mouse model exhibits intestinal pathology, specifically substrate inclusions in the myenteric plexus that mirror those reported in Fabry patients (34,39–41). We showed here that SRT is effective at limiting the amount of substrate accumulation in the intestines, especially when treatment was initiated early in life.
Because the biodistribution of an orally administered small molecule (Genz-682452) is different from that of an intravenously administered enzyme (rh α-Gal A), it was anticipated that the drugs would have differential effects in different tissues. Indeed, we noted that Genz-682452 had a more profound lipid-lowering effect in the kidney than rh α-Gal A. The decrease in GL-3 noted in mice treated with Genz-682452 likely resulted from lipid turnover and clearance by exosomal shedding from the tubular epithelial cells into the urine (42). Supporting this notion was the observation that the isoform pattern of GL-3 (differing relative abundance of ceramides with various acyl chain lengths) in urine was similar to that in kidney but not plasma (data not shown). This indicates that the source of urinary GL-3 was primarily from the kidney rather than plasma filtrate. Furthermore, substrate inclusions have been noted in the epithelial cells of the collecting ducts, distal tubules and proximal tubules as well as in parietal epithelial cells in the glomerular Bowman capsule of Fabry mice (34).
Another tissue compartment that was preferentially addressed by Genz-682452 was the CNS. Large proteinaceous entities such as the intravenously administered α-Gal A are excluded from the CNS by the blood-brain barrier. In this regard, Genz-682452, by virtue of its ability to cross the blood-brain barrier, may be anticipated to better address the cerebro-vascular and auditory deficiencies and perhaps the psychological manifestations reportedly associated with the disease (43). The finding that mice treated with Genz-682452 but not rh α-Gal A displayed a delayed loss of their thermal nociception suggests that SRT can reduce the glycolipid accumulation in peripheral nerve cells. Interestingly, mice treated with Genz-682452 effected only a modestly greater reduction in the number of vacuolated neurons in the DRG than those administered rh α-Gal A. This suggests that perhaps some enzyme was able to gain access to perineural and other nerve cells. Indeed, it should be noted that provision of very high systemic levels of rh α-Gal A (such as by gene therapy) can address the pathology in the DRG and improve the animals’ thermal nociception (21). However, the data here suggest that the small molecule, perhaps given its greater exposure in the peripheral and CNS was more effective. Moreover, improvements in small fiber neuropathy and neuropathic pain have been reported in patients treated with agalsidase β (11) and agalsidase α (14). Intervening early may be an important consideration, because the small fiber peripheral neuropathy is likely due to nerve fiber loss. Although not surrogates for small fiber neuropathy, both the thermosensory assay and DRG evaluation support a role for initiating treatment early to protect against further irreversible damage to the peripheral nervous system. The importance of early intervention has previously been emphasized by Fabry clinical trials (4,7,8), as well as other lysosomal storage disorders such as Pompe disease (44).
Taken together, the data described here demonstrated that Genz-682452, a novel, orally available and specific GCS inhibitor, when used in conjunction with ERT, could further reduce the burden of substrate accumulation beyond that achieved by either monotherapy. Moreover, the data also serve to emphasize the importance of early therapeutic intervention to protect against irreversible pathological changes in affected males with Type 1 “Classic” and Type 2 “Later-onset” Fabry disease.
Disclosure
The authors except RJ Desnick are employees and/or shareholders of Sanofi. RJ Desnick is a consultant for Amicus Therapeutics and holds founder stock. He receives royalties from Genzyme Corporation and Shire HGT.
References
Desnick RJ, Ioannou YA, Eng CM. 150: α-Galactosidase A Deficiency: Fabry Disease. In: The Online Metabolic and Molecular Bases of Inherited Disease [Internet]. Valle D, et al. (eds.) McGraw-Hill, [New York]. Available from: https://doi.org/ommbid.mhmedical.com/content.aspx?bookid=474§ionid=45374153
Schiffmann R, Ries M, Timmons M, Flaherty JT, Brady RO. (2006) Long-term therapy with agalsidase alfa for Fabry disease: safety and effects on renal function in a home infusion setting. Nephrol. Dial. Transplant. 21:345–354.
Germain DP, et al. (2007) Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J. Am. Soc. Nephrol. 18:1547–1557.
Banikazemi M, et al. (2007) Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann. Intern. Med. 146:77–86.
Wilcox WR, et al. (2004) Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am. J. Hum. Genet. 75:65–74.
Vedder AC, et al. (2007) Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One. 2:e598.
Eng CM, et al. (2001) A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am. J. Hum. Genet. 68:711–722.
Eng CM, et al. (2001) Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry’s disease. N. Engl. J. Med. 345:9–16.
Thurberg BL, et al. (2002) Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 62:1933–1946.
Tondel C, et al. (2013) Agalsidase benefits renal histology in young patients with Fabry disease. J. Am. Soc. Nephrol. 24:137–148.
Dutsch M, Hilz MJ. (2010) Neurological complications in Fabry disease. Rev. Med. Interne. 31Suppl 2:S243–S250.
Hilz MJ, Brys M, Marthol H, Stemper B, Dutsch M. (2004) Enzyme replacement therapy improves function of C-, Adelta-, and Abeta-nerve fibers in Fabry neuropathy. Neurology. 62:1066–1072.
Schiffmann R, et al. (2003) Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 28:703–710.
Uceyler N, et al., (2011) Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J. Peripher. Nerv. Syst. 16:304–314.
Smid BE, Hollak CE. (2014) A systematic review on effectiveness and safety of eliglustat tartrate for type I Gaucher disease. Expert Opin. Orphan Drugs. 5(2):523–529.
Desnick RJ, Schuchman EH, Astrin KH, Cheng SH. (2013) Chapter 28 — Therapies for Lysosomal Storage Diseases. In: Emery and Rimoin’s Principles and Practice of Medical Genetics [Internet]. Rimoin DL, Pyeritz RE, Korf B (eds.) Elsevier Ltd, Waltham (MA). Available from: https://doi.org/www.sciencedirect.com/science/article/pii/B9780123838346000367
McEachern KA, et al. (2007) A specific and potent inhibitor of glucosylceramide synthase for substrate inhibition therapy of Gaucher disease. Mol. Genet. Metab. 91:259–267.
Marshall J, et al. (2010) Substrate reduction augments the efficacy of enzyme therapy in a mouse model of Fabry disease. PLoS One. 5:e15033.
Marshall J, et al. (2013) A novel, selective and orally-available glucosylceramide synthase inhibitor for substrate reduction therapy of Fabry disease. Poster session presented at the 63rd Annual Meeting of The American Society of Human Genetics; Oct 22–26; Boston, MA. Abstract available from: https://doi.org/www.ashg.org/2013meeting/abstracts/fulltext/f130121098.htm
Wang AM, et al. (1996) Generation of a mouse model with a-galactosidase A deficiency. Am. J. Hum. Genet. 59:A208.
Ziegler RJ, et al. (2007) Correction of the biochemical and functional deficits in fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase A. Mol. Ther. 15:492–500.
Ziegler RJ, et al. (2008) Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum. Gene. Ther. 19:609–621.
Ioannou YA, Zeidner KM, Gordon RE, Desnick RJ. (2001) Fabry disease: preclinical studies demonstrate the effectiveness of alpha-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 68:14–25.
Coles L, Hay JB, Gray GM. (1970) Factors affecting the glycosphingolipid composition of murine tissues. J. Lipid. Res. 11:158–163.
McCluer RH, Deutsch CK, Gross SK. (1983) Testosterone-responsive mouse kidney glycosphingolipids: developmental and inbred strain effects. Endocrinology. 113:251–258.
Desnick RJ, Sweeley CC, Krivit W. (1970) A method for the quantitative determination of neutral glycosphingolipids in urine sediment. J. Lipid. Res. 11:31–37.
Desnick RJ, Dawson G, Desnick SJ, Sweeley CC, Krivit W. (1971) Diagnosis of glycosphingolipidoses by urinary-sediment analysis. N. Engl. J. Med. 284:739–744.
Zhu Y, et al. (2009) Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol. Ther. 17:954–963.
Franco LM, et al. (2005) Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther. 12:876–84.
Mingozzi F, et al. (2003) Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 111:1347–1356.
McEachern KA, et al. (2006) AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J. Gene. Med. 8:719–729.
Ziegler RJ, et al. (2004) AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol. Ther. 9:231–240.
Kaminsky P, et al. (2013) Multidimensional analysis of clinical symptoms in patients with Fabry’s disease. Int. J. Clin. Pract. 67:120–127.
Bangari DS, et al. (2015) alpha-Galactosidase A Knockout Mice: Progressive Organ Pathology Resembles the Type 2 Later-Onset Phenotype of Fabry Disease. Am. J. Pathol. 185:651–665.
Thurberg BL, et al. (2009) Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation. 119:2561–2567.
Itier JM, et al. (2014) Effective clearance of GL-3 in a human iPSC-derived cardiomyocyte model of Fabry disease. J. Inherit. Metab. Dis. 37:1013–1022.
Mistry PK, et al. (2015) Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. J. Amer. Med. Assoc. 313:695–706.
Cox TM, et al. (2015) Eliglustat versus imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. pii:S0140–6736(14)61841–9.
Jack CI, Morris AI, Nasmyth DG, Carroll N. (1991) Colonic involvement in Fabry’s disease. Postgrad. Med. J. 67:584–585.
Deniz K, et al. (2011) Colonic involvement in Fabry disease. Int. J. Surg. Pathol. 19:777–778.
Hoffmann B, Schwarz M, Mehta A, Keshav S. (2007) Gastrointestinal symptoms in 342 patients with Fabry disease: prevalence and response to enzyme replacement therapy. Clin. Gastroenterol. Hepatol. 5:1447–1453.
Gonzales P, Pisitkun T, Knepper MA. (2008) Urinary exosomes: is there a future? Nephrol. Dial. Transplant. 23:1799–1801.
Crosbie TW, Packman W, Packman S. (2009) Psychological aspects of patients with Fabry disease. J. Inherit. Metab. Dis. 32:745–753.
Chien YH, et al. (2011) Later-onset Pompe disease: early detection and early treatment initiation enabled by newborn screening. J. Pediatr. 158:1023–1027.e1.
Acknowledgments
The authors would like to thank Leah Curtin, Erik Zarazinski, Christina Norton, Amy Allaire, JoAnne Fagan and other members of the Comparative Medicine department for animal husbandry and assistance with animal studies. We also acknowledge the members of the Histology and Pathology departments for their expertise in processing the tissue samples, Nelson Yew and Malgorzata Przybylska for Molecular Biology, and Ray Gimi, Jin Zhao, Paul Konowicz, Mike Reardon and members of Chemistry for the synthesis and purification of Genz-682452.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (https://doi.org/creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Ashe, K.M., Budman, E., Bangari, D.S. et al. Efficacy of Enzyme and Substrate Reduction Therapy with a Novel Antagonist of Glucosylceramide Synthase for Fabry Disease. Mol Med 21, 389–399 (2015). https://doi.org/10.2119/molmed.2015.00088
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2015.00088