Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by abnormal function of both the innate and the adaptive immune system, leading to a loss of tolerance to self-antigens. Monocytes are a key component of the innate immune system and are efficient producers of multiple cytokines. In SLE, inappropriate activation of monocytes is thought to contribute to the loss of self-tolerance. In this study, we demonstrate that type 1 interferon (IFN) production by CpG-challenged monocytes can be suppressed by C1q through activating leukocyte-associated Ig-like receptor-1 (LAIR-1), which contains immunoreceptor tyrosine-based inhibition motifs (ITIMs). The phosphorylation of LAIR-1 and the interaction of LAIR-1 with SH2 domain-containing protein tyrosine phosphatase-1 (SHP-1) were enhanced after LAIR-1 engagement by C1q. Moreover, engagement of LAIR-1 by C1q inhibited nuclear translocation of interferon regulatory factor (IRF)-3 and IRF5 in CpG-stimulated monocytes. These data suggest a model in which LAIR-1 engagement by C1q helps maintain monocyte tolerance, specifically with respect to Toll-like receptor-9-mediated monocyte activation.
Similar content being viewed by others
Introduction
Early studies on the pathogenesis of systemic lupus erythematosus (SLE) focused on the adaptive immune system, since B and T lymphocyte abnormalities were thought to be the primary cause of autoimmunity. However, it is now increasingly recognized that components of the innate immune system also play an essential role in SLE (1–5).
Monocytes are myeloid cells that play a key role in innate immunity and are efficient producers of proinflammatory cytokines and type 1 interferons (IFNs), IFNα and IFNβ, when stimulated by pathogen-associated molecular patterns (PAMPs) such as unmethylated bacterial DNA or damage-associated molecular patterns (DAMPs) such as apoptotic debris (2,5,6). Numerous monocyte defects involving aberrant activation and dysregulation of cytokine production have been identified in SLE patients (1,3). Notably, increased levels of type 1 IFN are seen in virtually all pediatric patients and a substantial percentage of adult patients. High IFN levels are a feature of some unaffected first-degree relatives as well (7,8).
TLR9, expressed by B cells, macrophages, monocytes, dendritic cells (DCs) and plasmacytoid DCs (pDCs), recognizes CpG, which mimics bacterial DNA (9–13). CpG 2216 is a prototype of the class of CpG (CPG-A), which preferentially activates myeloid cells as opposed to B cells (14). Human monocytes exposed to CpG-A can differentiate into DCs and produce a number of cytokines including interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α and type I IFN (15). When TLR9 associates with CpG motifs in the endosome, it recruits MyD88; the TLR9/MyD88 complex leads to activation of interferon regulatory factors (IRFs) (16,17). IRFs including IRF3, IRF5 and IRF7 are phosphorylated and translocate into the nucleus, where they regulate transcription of type 1 IFN mRNA. IRF3 and IRF8 cooperatively regulate IFNβ production in monocytes stimulated with TLR ligands such as LPS (TLR4), Pam3csk4 (TLR2) or viral infection (18), whereas IRF3 cooperates with IRF7 to regulate IFNβ production in pDCs on TLR9 stimulation (19). Secreted type 1 IFNs bind to the IFN receptor (IFNR) acting in an autocrine manner to induce the expression of a set of secondary IFN response genes (IFN signature genes [ISGs]) such as Mx1 and OAS1 (20). Expression of these genes is tightly regulated by type 1 IFNs through the consensus IFN-stimulated response elements (20,21). IRF5 also regulates transcription of the proinflammatory cytokines IL-6 and TNFα (22); IRF5 and nuclear factor (NF)-κB p50 coregulate IL-6 in TLR9-stimulated human plasmacytoid DCs (pDCs) (23). Genetic polymorphisms of IRF3, IRF5 and IRF7 have been associated with susceptibility to SLE (17,24) and elevated levels of nuclear IRF5 have been demonstrated in monocytes of SLE patients (4).
A Src family kinase (SFK)-driven tyrosine phosphorylation pathway at the plasma membrane is upstream of and required for TLR9/MyD88 activation in endosomes (12). This result suggests that a potential CpG-sensing receptor is localized at the plasma membrane and may activate SFKs. Two SFKs, Hck and Lyn, are phosphorylated in monocytes after stimulation by CpG and induce actin cytoskeleton reorganization. They also activate the TLR9/MyD88 signaling cascade (12). The activation of SFKs is implemented through the catalytic activity of the kinase domain and through proteinprotein interactions of the regulatory SH2 and SH3 domains (25,26). Regulation of SFKs is modulated by C-Src kinase (Csk), which phosphorylates the C-terminal tyrosine of SFK, resulting in a catalytically inactive conformation (27). Although much is understood regarding the production of IFN downstream of TLR9, the membrane proximal molecular events that suppress these pathways to prevent overproduction of cytokines have not been well described.
Leukocyte-associated Ig-like receptor-1 (LAIR-1) is an inhibitory immune receptor with immunoreceptor tyrosine-based inhibition motifs (ITIMs). It is expressed on human myeloid and lymphoid cells, including NK cells, T cells, B cells and monocytes; monocyte-derived DCs; and CD34+ hematopoietic progenitor cells (28–32). LAIR-1 engagement inhibits the differentiation of peripheral blood precursors into DCs (33,34). On antibody-mediated cross-linking, the tyrosines in the ITIMs of LAIR-1 become phosphorylated; phosphorylation of both ITIMs is required for full inhibition of cellular activation (30,35). Phosphorylation of LAIR-1 is inhibited by PP2, a SFK inhibitor, suggesting that the kinase responsible for LAIR-1 phosphorylation is an SFK such as Lck, Hck or Lyn (36). Both human and mouse LAIR-1 are associated with Csk (30,35). Engagement of LAIR-1 recruits SH2 domain-containing protein tyrosine phosphatase-1 (SHP-1), which negatively regulates intracellular signaling (36). In NK cells, SHP-1 is associated with LAIR-1 upon stimulation by monoclonal anti-LAIR-1 antibody (31). Engagement of LAIR-1 by collagen recruits SHP-1, which negatively regulates intracellular signaling (36). SHP-1 appears to be important for negative regulation of type 1 IFN production and consequent protection against SLE-like inflammation (24,37).
The complement component C1q and extracellular matrix collagens are functional ligands for LAIR-1 and directly inhibit immune cell activation (34,38,39). C1q has been shown to regulate cytokine production and MyD88-dependent TLR-mediated signaling in murine bone marrow-derived DCs (40) and to inhibit IFNα production by human pDCs in response to stimulation by TLR ligands in vitro (34). Moreover, C1q deficiency has been associated with abnormal production of IFNα by pDCs in SLE patients (41).
Here, we set out to define how C1q suppresses CpG-mediated activation of monocytes. First, we demonstrate that CpG-induced production of type 1 IFN by monocytes is suppressed by C1q or anti-LAIR-1 monoclonal antibodies (mAbs) and is characterized by less nuclear translocation of IRFs. C1q-mediated suppression did not occur in cells transfected with LAIR-1 siRNA, confirming the importance of LAIR-1 to this inhibitory pathway. CpG alone augmented an interaction between LAIR-1 and the inactive form of Hck. However, when LAIR-1 was engaged by C1q or anti-LAIR-1 antibody in CpG-stimulated monocytes, the interaction between Hck and LAIR-1 was abrogated, and an interaction between SHP-1 and LAIR-1 was evident. Interestingly, we also found that LAIR-1 mediates a ligand-independent suppression of cytokine production. These findings demonstrate that C1q and LAIR-1 are dynamically involved in monocyte homeostasis.
Materials and Methods
Reagents
Fluorochrome-conjugated and unconjugated antibodies were purchased: phycoerythrin (PE)-labeled or unlabeled mouse anti-human LAIR-1 (DX26; BD Pharmingen [BD Biosciences, San Jose, CA, USA]) for flow cytometry or stimulation; goat anti-human LAIR-1 (T-15, Santa Cruz Biotechnology, Dallas, TX, USA) for Western blotting; mouse anti-SHP-1 (D-11, Santa Cruz Biotechnology); rabbit anti-Hck (Abcam, Cambridge, MA, USA); mouse anti-phosphoSrc (Invitrogen [Thermo Fisher Scientific Inc., Waltham, MA, USA]); rabbit anti-IRF3 (Cell Signaling Technology, Danvers, MA, USA); rabbit anti-IRF5 (Protein Tech, Chicago, IL, USA); mouse anti-phospho-IkBα (Ser32/36; Cell Signaling Technology); mouse anti-phospho-IkBα (Cell Signaling Technology); mouse anti-lamin A/C (Sigma-Aldrich, St. Louis, MO, USA); mouse anti-β actin (AC-15, Sigma-Aldrich); rabbit anti-GAPDH (anti-glyceraldehyde-3-phosphate dehydrogenase) (Cell Signaling Technology); anti-CD11b, anti-CD14 and isotype-matched control antibodies (BD Pharmingen [BD Biosciences]); and human Fc receptor blocking solution (Biolegend, San Diego, CA, USA). Cell culture reagents included the following: Ficoll-plaque plus (GE Healthcare, London, UK); penicillin/streptomycin, RPMI 1640, L-glutamine and HEPES (all from Gibco/Invitrogen [Thermo Fisher Scientific]); heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT, USA); and X-VIVO serum-free media (Lonza, Basel, Switzerland). Other reagents used included: C1q from Complement Technology (Tyler, TX, USA); CpG 2216 (Invitrogen [Thermo Fisher Scientific]); 1× RIPA cell lysis buffer (Invitrogen [Thermo Fisher Scientific]); protease inhibitor cocktail (Pierce, Waltham, MA, USA); phosphatase inhibitors (Pierce); formaldehyde, Triton X-100 and NP-40 (Sigma-Aldrich); Tween-20 (Fisher Scientific [Thermo Fisher Scientific]); and phosphate-buffered saline (PBS) and distilled water (Gibco/Invitrogen [Thermo Fisher Scientific]). Proteins and culture reagents were endotoxin-tested (<0.1 Endotoxin Units [EU]/mL) either by the manufacturer or by using a limulus amebocyte lysate assay kit performed per the manufacturer’s instructions (Charles River Endosafe, Kingston, NY, USA).
Human Monocyte Isolation, Culture and Stimulation
Human peripheral blood mononuclear cells (PBMCs), obtained according to institutional guidelines of the Feinstein Institute for Medical Research, were isolated from the blood of healthy donors by density centrifugation (New York Blood Center, New York, NY, USA). Monocytes were negatively enriched by using a human monocyte enrichment kit (Stemcell Technologies, Vancouver, BC, Canada). Purity of monocytes (∼90% CD11b+ CD14+) was assayed by flow cytometry. Purified monocytes (1 × 106 cells/mL) were immediately stimulated for indicated times until 30 min with CpG 2216 (5 µmol/L), C1q (25 µg/mL) or anti-LAIR-1 antibody (10 µg/mL) in X-VIVO serum-free medium and harvested at the indicated time points. For cytokine assay, cells were cultured in U-bottom 96-well plates (Nunc, Waltham, MA, USA) containing complete media (RPMI-1640 containing 2 mmol/L L-glutamine, 10 mmol/L HEPES, 50 IU/mL penicillin, 50 mg/mL streptomycin and 10% FBS).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted with an RNeasy kit (Qiagen, Hilden, Germany) and subjected to reverse transcription with an iScript cDNA synthesis kit (BioRad, Hercules, CA, USA). cDNA was analyzed by quantitative polymerase chain reaction (qPCR) by using a LightCycler 480 master mix with TaqMan probes (Applied Biosystems [Thermo Fisher Scientific]) against human IFNα4 (Hs01681284), IFNβ1 (Hs01077958), MX1 (Hs00182073), OAS1 (Hs00242942), IL-6 (Hs00985639), TNFα (Hs00174128) and Polr2A (Hs00172187). Data were normalized to Polr2a; relative induction was calculated by ΔΔCt.
Cytokine Assay
Cytokine analysis for IL-6 and TNFα was performed using a Human ProInflammatory 7-Plex Ultra-Sensitive Kit (Meso Scale Discovery [MSD], Rockville, MD). MSD plates were analyzed on the MS2400 imager (MSD). Assay was performed according to the manufacturer’s instructions. All standards and samples were measured in duplicate.
Isolation of Nuclear Fractions
The NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific [Thermo Fisher Scientific]) was used for nuclear extraction following the manufacturer’s protocol. The efficiency of nuclear fractions was determined by immunoblotting for lamin.
Confocal Microscopy
Unstimulated or stimulated monocytes were washed three times with ice-cold PBS, fixed with 4% (v/v) paraformaldehyde and permeablized with 0.2% Triton X-100. Cells were seeded (2 × 105 cells/mL) on slides by using cytospin and blocked with 2% goat serum and 2% bovine serum albumin (Invitrogen [Thermo Fisher Scientific]). Cells were incubated overnight at 4°C with anti-IRF3 antibody (1:100) and washed and incubated with Alexa Fluor 488-labeled goat anti-mouse antibody (1:200; Invitrogen [Thermo Fisher Scientific]). Nuclei were stained with propidium iodide (0.5 µg/mL; Sigma-Aldrich). Cells were washed and mounted, and confocal images were captured by FluoView 300 (Olympus, Center Valley, PA, USA) and analyzed by using Axio vision 4.8 software (Zeiss, Jena, Germany).
Transfection and Flow Cytometry
In RNA interference assays, monocytes were transfected by using an AmaxaNucleofector kit (Lonza). Transfection efficiency was over 40%. siRNAs for LAIR-1 or control siRNA were from Qiagen. The target sequence of human LAIR-1 is CAGCATCCAGAAGGTTCGTTA. The efficiency of knockdown was determined by flow cytometry (FACS verse; BD Biosciences) with PE-labeled anti-human LAIR-1 antibody. Data were analyzed by using FlowJo software (Tree Star; see https://doi.org/www.flowjo.com/).
Immunoprecipitation and Western Blot Analysis
Unstimulated or CpG-stimulated monocytes (2–5 × 106 cells/mL) were washed in ice-cold PBS and lysed in 1× RIPA buffer containing complete protease inhibitor mixture (Roche, Basel, Switzerland) and phosphatase inhibitor (Pierce). For immunoprecipitation, cell lysates were then diluted 10-fold with lysis buffer and anti-LAIR-1 antibody before being incubated with precleared protein G-dynabeads (Life Technologies [Thermo Fisher Scientific]). Immunoblotting of bound proteins was performed with the indicated antibodies.
Phospho-Immunoreceptor Array
To determine tyrosine phosphorylated LAIR-1, cell lysates from monocytes (7 × 106) stimulated without CpG or with CpG only, CpG plus C1q or CpG plus anti-LAIR-1 antibody were incubated with human phosphoimmunoreceptor array membranes (Proteome Profiler Array; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. Phosphorylation levels of individual analytes were determined by average pixel density of duplicate spots; values were obtained after subtraction of background and were normalized to positive control spots.
Statistical Analysis
Statistical analyses were performed with Prism 5.0 (GraphPad, La Jolla, CA, USA). P values were calculated by using a two-tailed unpaired Student t test or two-way ANOVA. P values of <0.05 were considered significant: *p < 0.05, **p < 0.01 and ≤ 0.001 (Student t test).
Results
C1q and LAIR-1 Modulate the Expression of Multiple Genes in Human Monocytes Stimulated with CpG
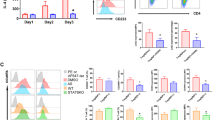
We have previously shown that the addition of C1q to human pDCs inhibited IFNα production after CpG stimulation in a dose-dependent fashion. In addition, this inhibition was reversed by soluble LAIR-2 (34), suggesting that the inhibitory effects of C1q required that C1q bind to LAIR-1. Besides pDCs, human monocytes also highly express LAIR-1 and are efficient producers of type 1 IFN and NF-κB-dependent cytokines after TLR9 ligation. We examined a potential role of the LAIR-1 signaling cascade activated by C1q or anti-LAIR-1 mAb in regulating the expression of type 1 IFNs (IFNα and IFNβ) and the ISGs MX1 and OAS1 in human monocytes. We stimulated monocytes in serum-free medium lacking C1q, with CpG ODN 2216, which activates TLR9 (14). Significantly, IFN and ISGs were induced by 4 h after CpG stimulation, but not after C1q stimulation alone. Stimulation with CpG together with C1q led to diminished transcription of IFN and downstream ISGs (Figures 1A, B). The proinflammatory cytokines IL-6 and TNFα were also induced by CpG exposure and inhibited at the mRNA and protein level in the presence of C1q (Figures 1C, D). Anti-LAIR-1 antibody added to CpG-stimulated cells, but not control mouse IgG, also inhibited induction of IFN, ISGs and proinflammatory cytokines, suggesting that LAIR-1 engagement might be the mechanism for C1q-induced suppression.
LAIR-1 engagement by C1q or anti-LAIR-1 suppresses CpG-triggered production of cytokines in human monocytes. Purified human monocytes from healthy donors were unstimulated or stimulated with CpGODN 2216 (CpG), C1q with CpG or anti-LAIR-1 antibody with CpG for 4 h. Transcripts were determined by qRT-PCR. C1q or anti-LAIR-1 antibody inhibited CpG-mediated IFNα and IFNβ induction (A), IFN signature genes MX1 and OAS1 (B) and CpG-mediated IL-6 and TNFα induction (C). (D) After 24 h of culture, supernatants were collected, and the production of IL-6 and TNFα was measured by MSD assay. Utx, untreated. R.E., relative expression. Results represent the means ± standard error of the mean (SEM); the data were obtained from three independent experiments. *P < 0.05, **P < 0.01, ***P< 0.001.
To confirm the importance of LAIR-1, we used LAIR-1 siRNA to decrease LAIR-1 expression (Figure 2C). While cells transfected with control siRNA responded to CpG with increased expression of IFNα and showed an inhibition of CpG activation in the presence of C1q or anti-LAIR-1 antibody, cells transfected with LAIR-1 siRNA exhibited a higher basal level of IFNα transcription and less inhibition by C1q or anti-LAIR-1 antibody. The fact that basal IFNα transcription was not increased in cells transfected with control siRNA suggests that the increase in IFNα transcription present in LAIR-1 siRNA-transfected monocytes was not due to engagement of TLRs by siRNA, but rather that LAIR-1 has a constitutive C1q-independent inhibitory function in monocytes. TNFα transcription was increased by CpG stimulation in monocytes transfected with control siRNA and was inhibited by C1q or anti-LAIR-1 antibody (Figure 2B). Basal TNFα transcription was increased in monocytes transfected with LAIR-1 siRNA, and there was no discernable increase on exposure to CpG; thus, there was no inhibition by C1q or anti-LAIR-1 antibody (Figure 2B). The data indicate that C1q and LAIR-1 have an important regulatory function in controlling cytokine production in response to CpG challenge in monocytes.
C1q-meditated suppression of IFNα and TNFα production is reversed by LAIR-1 siRNA. (A, B) Human monocytes were transfected with control siRNA or LAIR-1 siRNA. After 24 h, cells were stimulated with CpG, C1q, CpG plus C1q or CpG and anti-LAIR-1 antibody for 4 h. Transcripts of IFNα and TNFα were determined by qRT-PCR. Results represent mean ± SEM; the data were obtained from three independent experiments. *P< 0.05, **P< 0.01, ***P < 0.0001, ns, not significant. Percent inhibition was calculated as based on qRT-PCR. (C) The efficiency of siRNA was determined at 24 h by flow cytometry with PE-labeled anti-LAIR-1 antibody.
C1q and LAIR-1 Inhibit the Nuclear Translocation of IRFs Mediated by CpG Stimulation
Because the transcription of type 1 IFNs and ISGs is strongly regulated by IRFs, we studied the activation of these molecules after CpG stimulation of human monocytes. We isolated nuclear fractions from human monocytes that had been stimulated with CpG, with or without C1q or anti-LAIR-1 antibody. CpG caused an increased nuclear localization of IRF3 and IRF5, which was not seen in the presence of C1q or anti-LAIR-1 antibody (Figure 3A). We used confocal microscopy to confirm our observations on the subcellular localization of IRF3. In unstimulated monocytes or monocytes treated with C1q alone, IRF3 localized to the cytoplasm. When CpG was added to monocytes, almost all detectable IRF3 was translocated to the nucleus (Figure 3B). The effect of CpG on IRF3 nuclear translocation was suppressed by coincubation with C1q or anti-LAIR-1 antibody. Approximately 75% of cells retained cytoplasmic IRF3 in the presence of C1q (Figure 3B). As shown in Figure 3C, phosphorylation of IkBα, which releases the cytoplasmic retention of NF-κB, was enhanced by CpG stimulation and reduced after CpG stimulation in the presence of C1q or anti-LAIR-1 antibody. These data suggest that C1q and LAIR-1 signaling negatively regulates the nuclear translocation of the key transcription factors for cytokine gene activation. Nuclear IRFs bind to responsive elements in the promoters in type 1 IFN genes. Furthermore, IRFs cooperate with NF-κB to induce proinflammatory cytokines. Thus, C1q mediates suppression of monocytes to produce cytokines through these two important pathways.
LAIR-1 engagement inhibits CpG-mediated nuclear translocation of IRFs and the activation of NF-κB. Human monocytes were stimulated with CpG, C1q or anti-LAIR-1 antibody for 3 h. Nuclear fractions were analyzed by Western blot for IRF5, IRF3 and lamin for a marker for the nuclear fraction. C1q and anti-LAIR-1 antibody inhibit CpG-mediated nuclear translocation of IRF5 and IRF3 (A). Data are representative of three independent experiments. (B) Stimulated cells were stained for IRF3 and propodium iodine (PI) as a nuclear marker. The percentage of IRF3 nuclear translocation was assessed in more than 200 cells in three independent experiments, and representative images are shown. All images were viewed at 120× magnification by using a FluoView 300 confocal microscope (Olympus). (C) Purified monocytes were stimulated with CpG or C1q together with CpG for the indicated times, and cell lysates were assayed for phosphorylated-IkBα by Western blotting. Phosphorylated IkBα was normalized to GAPDH. Experiments were repeated three times, and representative data are shown.
LAIR-1 Sequesters the Inactive Hck during CpG-Mediated Activation While C1q Leads to SHP-1 Engagement with LAIR-1
We next asked how LAIR-1 negatively regulates CpG-mediated activation of monocytes. To determine which proteins might be associated with LAIR-1, we performed coimmunoprecipitation studies. We found that Csk is associated with LAIR-1 in unstimulated monocytes, and this association is present also in CpG-stimulated monocytes (Figure 4A). Because Csk is known to phosphorylate the inhibitory tyrosine of SFKs, we tested for SFK phosphorylation at this site. We found that inactive SFK was associated with LAIR-1 in the resting state and following CpG exposure (Figure 4A). We observed Hck to be the major binding partner for LAIR-1 in both unstimulated and CpG-stimulated monocytes. When LAIR-1 was engaged by C1q or anti-LAIR-1 antibody in CpG-stimulated monocytes, the interaction between Hck and LAIR-1 was abrogated, and an interaction between SHP-1 and LAIR-1 was enhanced (Figure 4A).
LAIR-1 engagement induces an interaction between LAIR-1 and SHP-1, but CpG stimulation induces an interaction of Hck with LAIR-1. (A) Human monocytes were stimulated with CpG, C1q, CpG plus C1q or CpG plus anti-LAIR-1 antibody for 30 min. Cell lysates were immunoprecipitated with anti-LAIR-1 antibody, and LAIR-1 binding proteins were analyzed by Western blot. Data are representative of three independent experiments. (B) Cells were stimulated for the indicated time, and the same experiment as in (A) was performed. Numbers in blots represent density, and relative bindings were calculated by the ratio for the immune-precipitated LAIR-1. Experiments were repeated three times, and representative data are shown.
To explore the dynamic interactions of these proteins, we performed the same coimmunoprecipitation study at different time points after CpG stimulation in the absence or presence of C1q (5),15 and 30 min). The results of this analysis suggested that Csk constitutively binds to LAIR-1. Hck binds to LAIR-1 and is inactivated, presumably by Csk, after CpG stimulation. However, on engagement by C1q, LAIR-1 changes binding partners and associates with SHP-1 (Figure 4B).
Next we studied whether CpG stimulation or LAIR-1 engagement alters the phosphorylation of LAIR-1. We previously reported that C1q, C1q collagen tail or anti-LAIR-1 antibody each induce phosphorylation of LAIR-1 (34). Here, monocytes were stimulated with CpG, CpG plus C1q or CpG plus anti-LAIR-1 antibody. There was detectable phosphorylation of LAIR-1 even in unstimulated monocytes, perhaps accounting for the constitutive inhibition mediated by LAIR-1. CpG challenge modestly increased LAIR-1 phosphorylation, whereas LAIR-1 engagement with C1q or anti-LAIR-1 antibody strongly induced LAIR-1 phosphorylation (Figure 5). Presumably, Hck is responsible for the phosphorylation of LAIR-1 that occurs after C1q binding.
LAIR-1 engagement induces the tyrosine phosphorylation. The state of LAIR-1 phosphorylation was measured by a proteome profile array-human phospho-immunoreceptor assay. Whole cell extracts from untreated (Utx), CpG, CpG plus C1q or CpG plus anti-LAIR-1 antibody were analyzed. The experiment was repeated three times, and representative data are shown. Relative quantification for the phosphorylation of LAIR-1 was normalized to control spots.
Discussion
In this study, we demonstrate a suppressive mechanism of C1q in human monocytes. Monocytes have been increasingly recognized to play a role in both the initiation and propagation of SLE given their functions in phagocytosis and cytokine production (1,5,6,42). In particular, in the pristane-induced model of murine lupus, monocytes play a key pathogenic role by acting as a major producer of type I IFNs (5). Human monocytes express TLR9 and respond to CpG (13,43). We observed that C1q or anti-LAIR-1 antibody inhibited CpG-mediated nuclear translocation of IRF3 and IRF5 and phosphorylation of IkBα. The fact that the suppression of CpG-mediated activation of monocytes by C1q was reversed by LAIR-1-specific siRNA demonstrated that the suppressive effects of C1q are LAIR-1 dependent. Transfection of monocytes with LAIR-1 siRNA led to higher baseline levels of cytokine transcription, demonstrating a role for LAIR-1 in monocyte homeostasis. Moreover, CpG did not increase cytokine expression in monocytes lacking LAIR-1 expression, suggesting that CpG stimulation blocks the constitutive inhibitory function of LAIR-1. Our findings demonstrate a contribution of C1q deficiency to SLE that is independent of the role of C1q in clearance of apoptotic debris.
To identify the proteins that interact with LAIR-1 after TLR9 activation with or without LAIR-1 engagement, we performed immunoprecipitation studies by using anti-LAIR-1 antibody. Csk constitutively associates with LAIR-1. We observed an increased association of inactive Hck with LAIR-1 upon CpG treatment; presumably Csk phosphorylates the C-terminal tyrosine of Hck to produce the inactive form that we observed. We propose that CpG activates Csk leading to phosphorylation of Y522 and inactivation of Hck on LAIR-1. Binding sites have been identified on LAIR-1 for Hck (Y251) (VTYAQL) and Csk (Y281) (ITYAAV). We also observed increased phosphorylation of the LAIR-1 ITIM motifs after CpG exposure, but phosphorylation was even greater in the presence of C1q or anti-LAIR-1 antibody. We propose that C1q engages LAIR-1, causing Hck to be activated, perhaps auto-catalytically, and leading to the phosphorylation of the ITIM motifs of LAIR-1. This step permits binding to SHP-1. SHP-1 binds to both ITIM motifs when they are phosphorylated. In this way, C1q and LAIR-1 mediate an inhibitory pathway modulating TLR9 activation (Figure 6). LAIR-1 mediates partial inhibition in a ligand-independent fashion. There may be some SHP-1 associated with LAIR-1 in the resting state. This association is not detected in our coimmunoprecipitation studies and is eliminated when CpG is present. After LAIR-1 engagement by C1q, phosphorylated LAIR-1 recruits SHP-1 and induces the phosphatase activity of SHP-1. SHP-1 dephosphorylates the activating tyrosine residues of SFKs, as well as various other intracellular tyrosine phosphorylation substrates.
Model demonstrating how LAIR-1 contributes to immune tolerance. (A) In the resting state, Csk constitutively associates with LAIR-1. (B) When LAIR-1 is engaged in the presence of C1q, Hck phosphorylates the ITIMs on LAIR-1. Phosphorylated LAIR-1 recruits SHP-1 and induces the phosphatase activity of SHP-1. SHP-1 mediates the inhibition of monocytes by dephosphorylating the activating tyrosine residues of various intracellular tyrosine-phosphorylation substrates and by inhibiting nuclear translocation of IRFs or NF-κB resulting in cytokine production.
IFNs are key mediators of host defense to viral and bacterial infection. They are also thought to be involved in the pathogenesis of SLE (17,20,44,45). Patients treated with IFN for hepatitis C infection develop anti-nuclear antibodies or overt SLE (46). Serum levels of IFN correlate with disease activity in SLE. We demonstrate that C1q and LAIR-1 signaling suppresses the activation of IRFs, and this mechanism regulates the transcription of multiple cytokines upon CpG stimulation in human monocytes.
Conclusion
An absence of C1q has been shown to lead to enhanced IFN production by both human and mouse pDCs (39,41). We also have tested whether C1q inhibits myeloid cell activation by other PAMPs such as LPS or high-mobility group protein B1 (HMGB1). Interestingly, C1q also has a suppressive function in the HMGB1-mediated production of IFN (unpublished data). Kanakoudi-Tskalidou et al. (7) showed that mean serum levels of HMGB1 are positively correlated with levels of IFNα and the expression of LAIR-1 on pDCs of SLE patients is significantly lower than its expression on pDCs of healthy controls (47). These findings suggest that C1q/LAIR-1 signaling mediates a major inhibitory pathway for the innate immune response. Manipulating LAIR-1 may be a strategy for regulating inflammation and, specifically, disease activity in SLE. Notably, we also have observed that the C1q collagen tail, which engages LAIR-1, inhibits TLR signaling. A recent report showed that collagenous domains of C-type lectin surfactant protein-D (SP-D) bind to LAIR-1 and regulate FcαR-mediated reactive oxygen production in neutrophils (48). These results suggest that LAIR-1 engagement by collagen-like domains may be a therapeutic strategy for controlling inflammation in SLE and inflammatory conditions.
Disclosure
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
Katsiari CG, Liossis SN, Sfikakis PP. (2010) The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: a reappraisal. Semin. Arthritis Rheum. 39:491–503.
Lee PY, et al. (2008) A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 180:5101–8.
Li Y, Lee PY, Reeves WH. (2010) Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch. Immunol. Ther. Exp. (Warsz). 58:355–64.
Stone RC, et al. (2012) Interferon regulatory factor 5 activation in monocytes of systemic lupus erythematosus patients is triggered by circulating autoantigens independent of type I interferons. Arthritis Rheum. 64:788–98.
Yang L, Feng D, Bi X, Stone RC, Barnes BJ. (2012) Monocytes from Irf5−/− mice have an intrinsic defect in their response to pristane-induced lupus. J. Immunol. 189:3741–50.
Hansmann L, Groeger S, von Wulffen W, Bein G, Hackstein H. (2008) Human monocytes represent a competitive source of interferon-alpha in peripheral blood. Clin. Immunol. 127:252–64.
Kanakoudi-Tsakalidou F, et al. (2014) Simultaneous changes in serum HMGB1 and IFN-alpha levels and in LAIR-1 expression on plasmatoid dendritic cells of patients with juvenile SLE: new therapeutic options? Lupus. 23:305–12.
Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. (2007) High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 8:492–502.
Ahmad-Nejad P, et al. (2002) Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958–68.
Bauer S, et al. (2001) Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U. S. A. 98:9237–42.
Hemmi H, et al. (2000) A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–5.
Sanjuan MA, et al. (2006) CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol. 172:1057–68.
Saxena M, Busca A, Pandey S, Kryworuchko M, Kumar A. (2011) CpG protects human monocytic cells against HIV-Vpr-induced apoptosis by cellular inhibitor of apoptosis-2 through the calcium-activated JNK pathway in a TLR9-independent manner. J. Immunol. 187:5865–78.
Kerkmann M, et al. (2003) Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 170:4465–74.
Shirota H, Klinman DM. (2012) Effect of CpG ODN on monocytic myeloid derived suppressor cells. Oncoimmunology. 1:780–2.
Gilliet M, Cao W, Liu YJ. (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8:594–606.
Salloum R, Niewold TB. (2011) Interferon regulatory factors in human lupus pathogenesis. Transl. Res. 157:326–31.
Li P, et al. (2011) IRF8 and IRF3 cooperatively regulate rapid interferon-beta induction in human blood monocytes. Blood. 117:2847–54.
Yang H, Ma G, Lin CH, Orr M, Wathelet MG. (2004) Mechanism for transcriptional synergy between interferon regulatory factor (IRF)-3 and IRF-7 in activation of the interferon-beta gene promoter. Eur. J. Biochem. 271:3693–703.
Delgado-Vega AM, Alarcon-Riquelme ME, Kozyrev SV. (2010) Genetic associations in type I interferon related pathways with autoimmunity. Arthritis Res. Ther. 12 Suppl 1:S2.
Honda K, Taniguchi T. (2006) IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644–58.
Takaoka A, et al. (2005) Integral role of IRF-5 in the gene induction programme activated by Tolllike receptors. Nature. 434:243–9.
Steinhagen F, et al. (2013) IRF-5 and NF-kappaB p50 co-regulate IFN-beta and IL-6 expression in TLR9-stimulated human plasmacytoid dendritic cells. Eur. J. Immunol. 43:1896–906.
Akahoshi M, et al. (2008) Promoter polymorphisms in the IRF3 gene confer protection against systemic lupus erythematosus. Lupus 17:568–74.
Schwartzberg PL. (1998) The many faces of Src: multiple functions of a prototypical tyrosine kinase. Oncogene. 17:1463–8.
Rhee I, Veillette A. (2012) Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat. Immunol. 13:439–47.
Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa H. (1991) CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J. Biol. Chem. 266:24249–52.
Bonaccorsi I, et al. (2010) The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNalpha production. PLoS One. 5:e15080.
van der Vuurst de Vries AR, Clevers H, Logtenberg T, Meyaard L. (1999) Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 29:3160–7.
Meyaard L. (2008) The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 83:799–803.
Meyaard L, et al. (1997) LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 7:283–90.
Tang X, et al. (2012) Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J. Immunol. 188:548–58.
Poggi A, Tomasello E, Ferrero E, Zocchi MR, Moretta L. (1998) p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur. J. Immunol. 28:2086–91.
Son M, Santiago-Schwarz F, Al-Abed Y, Diamond B. (2012) C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl. Acad. Sci. U. S. A. 109:E3160–7.
Verbrugge A, Ruiter Td T, Clevers H, Meyaard L. (2003) Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int. Immunol. 15:1349–58.
Xu M, Zhao R, Zhao ZJ. (2000) Identification and characterization of leukocyte-associated Ig-like receptor-1 as a major anchor protein of tyrosine phosphatase SHP-1 in hematopoietic cells. J. Biol. Chem. 275:17440–6.
Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J. (1998) Functional consequences of the SHP-1 defect in motheaten viable mice: role of NF-kappa B. Cell. Immunol. 185:49–58.
Elkon KB, Santer DM. (2012) Complement, interferon and lupus. Curr. Opin. Immunol. 24:665–70.
Lood C, et al. (2009) C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 60:3081–90.
Yamada M, et al. (2004) Complement C1q regulates LPS-induced cytokine production in bone marrow-derived dendritic cells. Eur. J. Immunol. 34:221–30.
Santer DM, et al. (2010) C1q deficiency leads to the defective suppression of IFN-alpha in response to nucleoprotein containing immune complexes. J. Immunol. 185:4738–49.
Byrne JC, et al. (2012) Genetics of SLE: functional relevance for monocytes/macrophages in disease. Clin. Dev. Immunol. 2012:582352.
Izaguirre A, et al. (2003) Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125–38.
Hooks JJ, et al. (1979) Immune interferon in the circulation of patients with autoimmune disease. N. Engl. J. Med. 301:5–8.
Banchereau J, Pascual V. (2006) Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 25:383–92.
Omagari K, et al. (2003) Anti-extractable nuclear antigens (ENA) antibodies in patients with chronic hepatitis C before and after treatment with interferon. Autoimmunity. 36:269–73.
Ma CY, et al. (2012) Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol. Int. 32:395–402.
Olde Nordkamp MJ, et al. (2014) Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J. Leukoc. Biol. 96:105–11.
Acknowledgments
This work was supported by National Institutes of Health Grant R01AR-049126 to B Diamond and an Arthritis Foundation Fellowship to M Son. We would like to thank Sylvia Jones for expert secretarial assistance and the flow cytometry and imaging cores of the Feinstein Institute for Medical Research and Barbara Sherry, Frances Santiago-Schwarz, Sun-Jung Kim and Yong-Rui Zou for review of the manuscript. We also thank Frances SantiagoSchwarz for initiating our studies of LAIR-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit (https://doi.org/creativecommons.org/licenses/by-nc-nd/4.0/)
About this article
Cite this article
Son, M., Diamond, B. C1q-Mediated Repression of Human Monocytes Is Regulated by Leukocyte-Associated Ig-Like Receptor 1 (LAIR-1). Mol Med 20, 559–568 (2014). https://doi.org/10.2119/molmed.2014.00185
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/molmed.2014.00185