Abstract
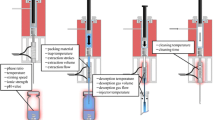
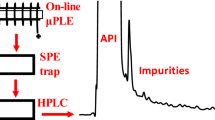
The purpose of analytical extractions is to simplify sample matrix without losing analyte molecules. Here we present a technique of extracting volatile compounds by scavenging gas-phase molecules with tiny liquid droplets (<10 μm). A cool mist of the extracting solvent is generated by an ultrasonic transducer, transferred to the headspace of the sample chamber under atmospheric conditions, and pushed by a small pressure difference toward a condenser. By slowly passing over the sample, the microdroplets extract volatile species present in the sample headspace, and they coalesce in a cooled zone. The condensed liquid is collected for analysis by direct infusion mass spectrometry or chromatography coupled with mass spectrometry. Due to the high surface-to-volume ratio of the microdroplets, the mist depletes a great share of the volatile organics present in the headspace. Other advantages of cool mist scavenging include: selective extraction of gas-phase molecules, the extracting solvent can be miscible with the sample solvent, simplicity, high speed, and no requirement for heating that could potentially decompose the sample. In this study, cool mist scavenging was first tested on artificial samples containing esters. The relationship between the sample concentration and the extract concentration was verified theoretically and experimentally. Some of the possible confounding effects were tested and discussed. The technique was subsequently applied to qualitative analysis of selected complex samples in liquid and solid phase as well as an esterification reaction.
Similar content being viewed by others
References
J. Pawliszyn (ed.), “Comprehensive Sampling and Sample Preparation”, 1st ed., 2012, Academic Press, Cambridge.
S. Liu and P. K. Dasgupta, Anal. Chem., 1995, 67, 2042.
H. Liu and P. K. Dasgupta, TrAC, Trends Anal. Chem., 1996, 75, 468.
A. Jain and K. K. Verma, Anal. Chim. Acta, 2011, 706, 37.
L. Guo, N. Nawi, and H. K. Lee, Anal. Chem., 2016, 88, 8409.
S. Jahan, Q. Zhang, A. Pratush, H. Xie, H. Xiao, L. Fan, and C. Cao, Anal. Chem., 2016, 88, 10490.
B. Ervens, P. Herckes, G. Feingold, T. Lee, J. L. Collett Jr., and S. M. Kreidenweis, J. Atmos. Chem., 2003, 46, 239.
P. Herckes, H. Chang, T. Lee, and J. L. Collett III, Water, Air, Soil Pollut., 2007, 787, 65.
S. Banerjee, E. Gnanamani, X. Yan, and R. N. Zare, Analyst, 2017, 742, 1399.
X. Chen, J. Cheng, and X. Yin, Proc. SPIE, 2003, 5058, 181.
I. A. Kaipper, L. A. S. Madureira, and H. X. Corseuil, J. Braz. Chem. Soc., 2001, 72, 514.
J. González-Sálamo, A. V. Herrera-Herrera, C. Fanali, and J. Hernández-Borges, LCGC Europe, 2016, 29, 180.
N. Yanase, H. Naganawa, T. Nagano, and J. Noro, Anal. Sci., 2011, 27, 171.
W. Henry, Philos. Trans. R. Soc. London, 1803, 93, 29.
L. Wang, Z. Wang, H. Zhang, X. Li, and H. Zhang, Anal. Chim. Acta, 2009, 647, 72.
K. Chingin, Y. Cai, J. Liang, and H. Chen, Anal. Chem., 2016, 88, 5033.
T. Wang and R. Lenahan, Bull. Environ. Contam. Toxicol., 1984, 32, 429.
C.-H. Chang and P. L. Urban, Anal. Chem., 2016, 88, 8735.
D. Rood, J. Chromatogr. Sci., 1997, 35, 187.
J.-B. Hu, S. Gunathilake, Y.-C. Chen, and P. L. Urban, RSC Adv., 2014, 4, 28865.
A. Schumpe and W.-D. Deckwer, Biotechnol. Bioeng., 1979, 27, 1075.
N. Segatin and C. Klofutar, Monatsh. Chem., 2000, 737, 131.
R. P. Schwarzenbach, P. M. Gschwend, and D. M. Imboden, “Environmental Organic Chemistry”, 1993, Wiley, New York.
V. Jakhetia, R. Patel, P. Khatri, N. Pahuja, S. Garg, A. Pandey, and S. Sharma, J. Adv. Sci. Res., 2010, 7, 19.
Acknowledgments
We thank Mr. Gurpur Rakesh D. Prabhu for discussions. We also acknowledge the Ministry of Science and Technology of Taiwan (Grant No.: MOST 104-2628-M-009-003-MY4) for financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, PC., Dutkiewicz, E.P. & Urban, P.L. Cool Mist Scavenging of Gas-Phase Molecules. ANAL. SCI. 33, 1161–1167 (2017). https://doi.org/10.2116/analsci.33.1161
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.33.1161