Abstract
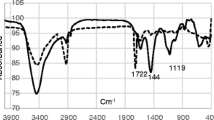
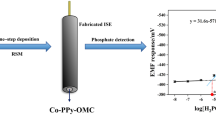
A phosphate ion-selective electrode using molybdenum metal was constructed. The modified molybdenum electrode responded to HPO42- in the presence of molybdenum dioxide and molybdophosphate (PMo12O403-) on the surface. The electrode exhibited a linear response to HPO42- in the concentration range between 1.0 × 10 5 and 1.0 × 10 1 M (mol dm−3) in the pH range from 8.0 to 9.5 with a detection limit of 1.0 × 10−6 M. The sensor showed near Nernstian characteristics (27.8 ± 0.5 mV dec−1) at pH 9.0. Since the responding potential was attributed to the activity of HPO42-, the potential at a given concentration of phosphate depended on the pH. The electrode indicated a good selectivity with respect to other common anions such as NO3-, SO42-, Cl-, HCO3- and CH3COO. The modified molybdenum electrode can be continuously used for over a 1 month with good reproducibility. The feasibility of the electrochemical sensor was proved by successful for the detection of phosphate in real samples.
Similar content being viewed by others
References
C. M. McGraw, S. E. Stitzel, J. Cleary, C. S. Slater, and D. Diamond, Talanta, 2007, 71, 1180.
C. Warwick, A. Guerreiro, and A. Soares, Biosens. Bioelectron., 2013, 41, 1.
P. Worsfold, I. McKelvie, and P. Monbet, Anal. Chim. Acta, 2016, 918, 8.
G. Nziguheba and E. Smolders, Sci. Total Envirom., 2008, 309, 53.
K. G. Raghothama, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1999, 50, 665.
K. G. Raghothama and A. S. Karthikeyan, Plant Soil, 2005, 274, 37.
E. M. Bennett, S. R. Carpenter, and N. F. Caraco, Bioscience, 2001, 51, 227.
S. R. Carpenter, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10002.
D. J. Conley, H. W. Pearl, R. W. Howarth, D. F. Boesch, S. P. Seitzinger, K. E. Havens, C. Lancelot, and G. E. Likens, Science, 2009, 323, 1014.
S. Savci, Int. J. Environ. Sci. Dev., 2012, 3, 77.
P. Manghat, R. Sodi, and R. Swaminathan, Ann. Clin. Biochem., 2014, 51, 631.
M. Copland, P. Komenda, E. D. Weinhandl, P. A. McCullough, and J. A. Morfin, Am. J. Kidney Dis., 2016, 68, S24.
S.-Y. Jung, J. Kwon, S. Park, J.-H. Jhee, H.-R. Yun, H.-N. Kim, Y.-K. Kee, C.-Y. Yoon, T.-I. Chang, E.-W. Kang, J.-T. Park, T.-H. Yoo, S.-W. Kang, and S.-H. Han, Plos One, 2018, 13, 1.
J. Li, L. Wang, M. Han, Y. Xiong, R. Liao, Y. Li, Si Sun, A. Maharjan, B.-H. Su, Nutrition and Diabetes, 2019, 9, 14.
G. F. Kirkbright and M. Marshall, Anal. Chem., 1973, 45, 1610.
W. Shotyk, J. Chromatogr., 1993, 640, 309.
Z.-L. Chen, P. Grierson, and M. A. Adams, Anal. Chim. Acta, 1998, 363, 191.
A. T. Lawal and S. B. Adeloju, Talanta, 2013, 114, 191.
B. Shyla and M. G. Nagendrappa, Spectrochim. Acta, Part A, 2011, 78, 497.
J. J. Wang and P. L. Bishop, Environ. Pollut., 2010, 158, 3612.
S. O. Engblom, Biosens. Bioelectron., 1998, 13, 981.
S. Berchmans, T. B. Issa, and P. Singh, Anal. Chim. Acta, 2012, 729, 7.
F. Tafesse and M. Enemchukwu, Talanta, 2011, 83, 1491.
D. Xiao, H.-Y. Yuan, J. Li, and R.-Q. Yu, Anal. Chem., 1995, 67, 288.
R. K. Meruva and M. E. Meyerhoff, Anal. Chem., 1996, 68, 2022.
K. Xu, Y. Kitazumi, K. Kano, and O. Shirai, Electrochim. Acta, 2018, 282, 242.
Y. Li, T. Jiang, X. Yu, and H. Yang, J. Electrochem. Soc., 2016, 163, B479.
D. Talarico, F. Arduini, A. Amine, D. Moscone, and G. Palleschi, Talanta, 2015, 141, 267.
A. F. Povey and A. A. Metcalfe, J. Electroanal. Chem., 1977, 84, 73.
F. Kivlehan, W. J. Mace, H. A. Moynihan, and D. W. N. Arrigan, Electrochim. Acta, 2009, 54, 1919.
V. S. Saji and C.-W. Lee, ChemSusChem, 2012, 5, 1146.
P. Wang, L. L. Wilson, D. J. Wesolowski, J. Rosenqvist, and A. Anderko, Corros. Sci., 2010, 52, 1625.
M. N. Hull, J. Electroanal. Chem., 1972, 38, 143.
Q. Xi, J. Liu, Z. Wu, H. Bi, Z. Li, K. Zhu, J. Zhuang, J. Chen, S. Lu, Y. Huang, and G. Qian, Appl. Surf. Sci., 2019, 480, 427.
A. G. Fogg and N. K. Bsebsu, Analyst, 1981, 1260, 369.
T. Tanaka, M. Miura, and T. Ishiyama, J. Trace Microprobe Tech., 2001, 19, 591.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xu, K., Kitazumi, Y., Kano, K. et al. Fabrication of a Phosphate Ion Selective Electrode Based on Modified Molybdenum Metal. ANAL. SCI. 36, 201–205 (2020). https://doi.org/10.2116/analsci.19P296
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.19P296