Abstract
Objectives
Several strategies have been proposed to manage the utilization of blood glucose test strips (BGTS) in Canada; however their potential impacts on utilization and costs of publically funded test strips are unknown.
Methods
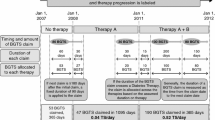
We investigated the impact of three potential policies that would restrict the number of test strips reimbursed by the public drug plans in Ontario and British Columbia (BC), and incorporated negotiated price reductions. These policies were based on recommendations from the Canadian Agency for Drugs and Technologies in Health, a briefing document by the Canadian Diabetes Association, and a new policy introduced by the Ontario Ministry of Health and Long-Term Care. BGTS utilization rates were assessed in two cross-sectional analyses among adults aged 18 years or older in BC and 65 or older in Ontario who received publicly-funded BGTS between January 2004 and December 2012. We modeled the 5-year utilization and cost implications of the three policies using time-series analysis.
Results
In 2012, there were 31 7,1 30 test strip recipients in Ontario and 1 36,659 recipients in BC, at a cost of $104.4 million and $22.6 million respectively. Under the scenarios of reduced BGTS quantities, 5-year cost savings ranged between $98.8 million (18.2% reduction) and $224.1 million (41.4% reduction) in Ontario and between $23.1 million (19.2% reduction) and $51.1 million (42.4% reduction) in BC. Price reductions of 15% resulted in annual savings of $14.4 million (1 3.7% reduction) in Ontario and $3.4 million (14.1% reduction) in BC.
Conclusions
Policies that align with evidence and expert guidance could impart substantial cost savings in multiple jurisdictions despite different public drug plans.
Résumé
Objectifs
Plusieurs stratégies ont été proposées pour gérer l’utilisation des bandelettes de test glycémique (BTG) au Canada, mais on ignore leurs effets potentiels sur l’utilisation et le coût des bandelettes de test glycémique financées par les deniers publics.
Méthodes
Nous avons étudié l’effet de trois politiques possibles qui limiteraient le nombre de bandelettes de test remboursées par les régimes d’assurance médicament en Ontario et en Colombie-Britannique (C.-B.), et avons incorporé des réductions de prix négociées. Ces politiques étaient fondées sur des recommandations de l’Agence canadienne des médicaments et des technologies de la santé, sur un document préparatoire de l’Association canadienne du diabète et sur une nouvelle politique introduite par le ministère de la Santé et des Soins de longue durée de l’Ontario. On a évalué le taux d’utilisation des BTG dans deux analyses croisées chez des adultes âgés de 18 ans et plus en C.-B. et de 65 ans et plus en Ontario qui recevaient des BTG financées par les fonds publics entre janvier 2004 et décembre 2012. Nous avons établi le modèle d’une utilisation sur 5 ans et des conséquences financières de ces trois politiques selon une analyse des séries chronologiques.
Résultats
En 2012, il y avait 31 7 1 30 receveurs de bandelettes en Ontario et 1 36 659 en C.-B., pour un coût de 104,4 millions $ et de 22,6 millions $ respectivement. Selon les scénarios des quantités réduites de BTG, les économies sur 5 ans étaient entre 98,8 millions $ (réduction de 18,2 %) et 224,1 millions $ (réduction de 41,4 %) en Ontario et entre 23,1 millions $ (réduction de 19,2 %) et 51,1 millions $ (réduction de 42,4 %) en C.-B. Une réduction de prix de 15 % entraînait des économies annuelles de 14,4 millions $ (réduction de 13,7 %) en Ontario et de 3,4 millions $ (réduction de 14,1 %) en C.-B.
Conclusions
Les politiques qui correspondent aux faits et aux conseils des experts pourraient permettre des économies importantes dans plusieurs compétences malgré les régimes publics d’assurance médicament différents.
Similar content being viewed by others
References
Public Health Agency of Canada. Diabetes in Canada: Facts and Figures from a Public Health Perspective. Ottawa, ON: PHAC, 2011.
Gomes T, Juurlink DN, Shah BR, Paterson JM, Mamdani MM. Blood glucose test strips: Options to reduce usage. CMAJ 2010;182(1):35–38. PMID: 20026624. doi: 10.1503/cmaj.091017.
Gomes T, Juurlink DN, Shah BR, Paterson JM, Mamdani MM. Blood Glucose Test Strip Use: Patterns, Costs and Potential Cost Reduction Associated with Reduced Testing. ICES Investigative Report. Toronto, ON: Institute for Clinical Evaluative Sciences, 2009.
O’Kane MJ, Bunting B, Copeland M, Coates VE. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): Randomised controlled trial. BMJ 2008;336(7654):1174–77. PMID: 18420662. doi: 10.1136/bmj.39534.571644.BE.
Clar C, Barnard K, Cummins E, Royle P, Waugh N. Self-monitoring of blood glucose in type 2 diabetes: Systematic review. Health Technol Assess 2010; 14(12)1–140. PMID: 20226138. doi: 10.3310/htal4120.
Agency for Drugs and Technologies in Health. Systematic Review of Use of Blood Glucose Test Strips for the Management of Diabetes Mellitus. 2009. Available at: https://doi.org/www.cadth.ca/media/pdf/BGTS_SR_Report_of_Clinical_Outcomes.pdf (Accessed February 14, 2015).
Agency for Drugs and Technologies in Health. Optimal Therapy Recommendations for the Prescribing and Use of Blood Glucose Test Strips. 2009. Report No. 3(6).
Miller D, Berard L, Cheng A, Hanna A, Hagerty D, Knip A. Self-monitoring of blood glucose in people with type 2 diabetes: Canadian Diabetes Association briefing document for healthcare providers. Can J Diabetes 2011;35:317–19. doi:10.1016/S1499-2671(11)54003-1.
Reimbursement levels for Blood Glucose Test Strips. Ontario Public Drug Programs. 2013. Available at: https://doi.org/www.health.gov.on.ca/en/pro/programs/drugs/teststrips/bg_teststrips.aspx (Accessed February 14, 2015).
Daw JR, Morgan SG. Stitching the gaps in the Canadian public drug coverage patchwork? A review of provincial pharmacare policy changes from 2000 to 2010. Health Policy 2012;104(1):19–26. PMID: 21978939. doi: 10.1016/j.healthpol.2011.08.015.
Medicine Prices Review Board. The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008. Ottawa, ON: PMPRB, 2013. Catalogue #: H82-13/2013E-PDF
Pharmaceutical Schedule. Pharmac: Pharmaceutical Management Agency. 2014. Available at: https://doi.org/www.pharmac.govt.nz/patients/Pharmaceutical Schedule/Schedule (Accessed February 14, 2015).
Pindyck RS, Rubinfeld DL. Econometric Models and Economic Forecasts. 4th Ed. New York, NY: Irwin McGraw-Hill, 1997.
Thongsai S, Youjaiyen M. The long-term impact of education on diabetes for older people: A systematic review. Glob J Health Sci 2013;5(6):30–39. PMID: 24171871. doi: 10.5539/gjhs.v5n6p30.
Steinsbekk A, Rygg LO, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res 2012;12(1):213. PMID: 22824531. doi: 10.1186/1472-6963-12-213.
Loveman E, Frampton GK, Clegg AJ. The clinical effectiveness of diabetes education models for Type 2 diabetes: A systematic review. Health Technol Assess 2008;12(9):1–116, iii. PMID: 18485272. doi: 10.3310/htal2090.
International Working Group. Self-monitoring of blood glucose in type 2 diabetes: An inter-country comparison. Diabetes Res Clin Pract 2008;82(3):e15–18. PMID: 18995920. doi: 10.1016/j.diabres.2008.08.021.
Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: Open parallel group randomised trial. BMJ 2007;335(7611):132. PMID: 17591623. doi: 10.1136/bmj.39247.447431.BE.
Davis WA, Bruce DG, Davis TM. Does self-monitoring of blood glucose improve outcome in type 2 diabetes? The Fremantle Diabetes Study. Diabetologia 2007;50(3):510–15. PMID: 17237940. doi: 10.1007/s00125-006-0581-0.
Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: A blinded, randomized trial. Am J Med 2005;118(4): 422–25. PMID: 15808142. doi: 10.1016/j.amjmed.2004.12.006.
-Insured Health Benefits (NIHB) Program Update. Health Canada. 2013. Available at: https://doi.org/qalipu.ca/site/wp-content/uploads/2014/02/Diabetic-Test-Strips-NIHB-update-for-clients-FINAL-2013-10-16.pdf (Accessed February 14, 2015).
Summary: April 2010 - March 2011. Drug Evaluation Alliance of Nova Scotia. 2011. Available at: https://doi.org/novascotia.ca/dhw/pharmacare/documents/deans/Annual_Summary_April_2011.pdf (Accessed February 14, 2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest: Dr. Muhammad Mamdani has received honoraria from Boehringer Ingelheim, Pfizer, Sanofi, Bristol-Myers Squibb, Astra-Zeneca, ClaxoSmithKline, Novo-Nordisk, Eli Lilly, Merck, and Bayer. Michael Law has consulted for Health Canada on unrelated pharmaceutical policy research. All other authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Gomes, T., Martins, D., Cheng, L. et al. The impact of policies to reduce blood glucose test strip utilization and costs in Canada. Can J Public Health 106, e210–e216 (2015). https://doi.org/10.17269/cjph.106.4788
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.17269/cjph.106.4788