Abstract
Background
Lymphadenectomy is currently the standard treatment for melanoma patients with palpable lymph node (LN) metastasis. There is no recommendation as to the time when surgery should be performed in France.
Objectives
The aim of this retrospective study was to assess the impact of the time interval preceding lymphadenectomy on patient outcomes.
Materials & methods
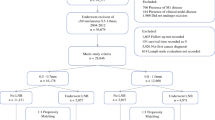
Patients who underwent lymphadenectomy for LN macrometastasis (Stage IIIB/C AJCC) between 2005 and 2012 were included. Both the time interval between the first suspicion of LN recurrence (physical examination and imaging) and lymphadenectomy and the time interval between the multidisciplinary team meeting and lymphadenectomy were recorded. The impact of these time intervals on patient relapse-free survival (RFS) and overall survival (OS) were analysed. The regression optimized (ROP) model was used to identify ghost factors for the Cox model.
Results
A total of 154 patients were included. The median time interval between the multidisciplinary team meeting and lymphadenectomy was 22 days (IQR: 6 to 66). The median time interval between the first suspicion of LN recurrence and lymphadenectomy was 59 days (IQR: 15 to 676). Taking into account the effect identified by the regression optimized (ROP) model, these times were associated with an increased risk of recurrence and mortality (p<0.001 and p = 0.01, respectively).
Conclusion
Our study demonstrates that increasing the time interval preceding lymphadenectomy significantly reduces patient RFS and OS.
Similar content being viewed by others
References
Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma European consensus-based interdisciplinary guideline-Update 2012. Eur J Cancer 2012; 48: 2375–90.
Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199–206.
Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial WHO Melanoma Programme. Lancet 1998; 351: 793–6.
Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127: 392–9.
Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 2014; 370: 599–609.
Nguyen JM, Gaultier A, Antonioli D. Abilities of statistical models to identify subjects with ghost prognosis factor. J Health Edu Res Dev 2015; 3:141.
Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19: 716–23.
Pizarro Á. Models of melanoma spread and final results of the Multicenter Selective Lymphadenectomy Trial-I. Actas Dermo-Sifiliográficas 2015; 106: 82–5.
Gershenwald JE, Colome MI, Lee JE, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998; 16: 2253–60.
Kettlewell S, Moyes C, Bray C, et al. Value of sentinel node status as a prognostic factor in melanoma: prospective observational study. BMJ 2006; 332: 1423.
Ryan M, Crow J, Kahmke R, Fisher SR, Su Z, Lee WT. FoxP3 and indoleamine 2,3-dioxygenase immunoreactivity in sentinel nodes from melanoma patients. Am J Otolaryngol 2014; 35: 689–94.
Medalie N, Ackerman AB. Sentinel node biopsy has no benefit for patients whose primary cutaneous melanoma has metastasized to a lymph node and therefore should be abandoned now. Br J Dermatol 2004; 151: 298–307.
Braun S, Cevatli BS, Assemi C, et al. Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J Clin Oncol 2001; 19: 1468–75.
Fisher B, Fisher ER. Barrier function of lymph node to tumor cells and erythrocytes I. Normal nodes. Cancer 1967; 20: 1907–13.
Kretschmer L, Hilgers R, Möhrle M, et al. Patients with lymphatic metastasis of cutaneous malignant melanoma benefit from sentinel lymphonodectomy and early excision of their nodal disease. Eur J Cancer 2004; 40: 212–8.
Tejera-Vaquerizo A, Nagore E, Puig S, et al. Effect of time to sentinel-node biopsy on the prognosis of cutaneous melanoma. Eur J Cancer 2015; 51: 1780–93.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Vildy, S., Nguyen, JM., Gaultier, A. et al. Impact of the time interval between lymph node recurrence and lymphadenectomy on melanoma patient survival. Eur J Dermatol 27, 166–173 (2017). https://doi.org/10.1684/ejd.2016.2955
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1684/ejd.2016.2955