Abstract
Background
Myricetin is a naturally occurring antioxidant commonly found in various plants. However, little information is available with respect to its direct anti-obesity effects.
Objective
This study was undertaken to investigate the effect of myricetin on high-fat diet (HFD)-induced obesity in C57BL/6 mice.
Results
Administration of myricetin dramatically reduced the body weight of diet-induced obese mice compared with solely HFD-induced mice. Several parameters related to obesity including serum glucose, triglyceride, and cholesterol were significantly decreased in myricetin-treated mice. Moreover, obesity-associated oxidative stress (glutathione peroxidase (GPX) activity, total antioxidant capacity (T-AOC), and malondialdehyde (MDA)) and inflammation (tumor necrosis factor-α (TNF-α)) were ameliorated in myricetin-treated mice. Further investigation revealed that the protective effect of myricetin against HFD-induced obesity in mice appeared to be partially mediated through the down-regulation of mRNA expression of adipogenic transcription factors peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα), and lipogenic transcription factor sterol regulatory element-binding protein 1c (SREBP-1c).
Conclusions
Consumption of myricetin may help to prevent obesity and obesity-related metabolic complications.
中文概要
目 的
研究杨梅素(myricetin)对高脂膳食(HFD)诱导的C57BL/6 小鼠肥胖的干预作用, 并探讨其作用机理。
创新点
首次在高脂膳食诱导的C57BL/6 肥胖小鼠模型中证明杨梅素可明显减轻肥胖小鼠的体重, 同时改善伴随肥胖的氧化应激和炎症。分子机理研究表明杨梅素通过下调脂代谢相关的转录因子(PPARγ、C/EBPα 和SREBP-1c)mRNA 的表达, 从而发挥减肥功效。
方 法
将C57BL/6 小鼠分为正常膳食组(ND, n=12)和高脂膳食组(HFD, n=24), 分别给予正常膳食和高脂膳食喂食2 周。之后, 高脂膳食组分为高脂膳食组(n=12)和杨梅素保护组(HFD+M, 灌胃150 mg/(kg·d)的杨梅素, n=12), 继续处理10 周。分析小鼠常规生理指标(体重和脏器重量)、生化指标(血糖浓度、甘油三酯和总胆固醇含量)、氧化应激指标(谷胱甘肽过氧化物酶(GPX)、超氧化物歧化酶(SOD)和总抗氧化能力(T-AOC))和炎症指标(肿瘤坏死因子α (TNF-α))等。苏木精-伊红染色法(H&E)和油红染色法分别观察脂肪组织和肝脏组织形态。实时逆转录聚合酶链反应(RT-PCR)分析小鼠脂肪组织PPARγ、C/EBPα 和SREBP-1c 等基因的mRNA 表达水平。
结 论
与高脂膳食对照组相比, 杨梅素处理可以显著减轻肥胖小鼠的体重。同时能显著降低肥胖小鼠血糖、甘油三酯和总胆固醇的含量。此外, 杨梅素处理能缓解伴随肥胖的氧化应激(GPX 活性、T-AOC 和丙二醛(MDA))和炎症(TNF-α)。分子机理研究发现杨梅素通过下调脂肪细胞生成(PPARγ 和C/EBPα)和脂质合成(SREBP-1c)密切相关的转录因子的mRNA 表达。本研究结果将为杨梅素减肥产品的开发提供一定的理论依据。
Similar content being viewed by others
1 Introduction
The World Health Organization (WHO) has estimated that globally more than 1.4 billion individuals are overweight or obese (Ng et al., 2014). A growing body of evidence confirms that obesity is closely associated with a series of chronic diseases, such as type 2 diabetes mellitus, cardiovascular diseases, nonalcoholic fatty liver disease (NAFLD), dyslipidemia, and certain cancers (Grundy, 1998; Tilg and Moschen, 2006). The high prevalence of obesity has made it a serious public health concern. Several factors including genetic, environmental, and psychological factors greatly influence the occurrence and development of obesity. Recently, increasing attention has been paid to exploiting natural components extracted from a normal diet as a viable strategy to inhibit the onset of obesity (Yun, 2010; Jeong et al., 2014). Some blended phytochemicals from fruits and vegetables reportedly have the potential to suppress diet-induced obesity (Song et al., 2013; Zhao et al., 2015).
Oxidative stress has been implicated in the development of obesity-related metabolic disorders, such as cardiovascular diseases, atherosclerosis, and hypertension (Furukawa et al., 2004; Galinier et al., 2006). Antioxidant defense systems may be impaired as a consequence of excessive oxidative stress in obesity (Furukawa et al., 2004). In addition, obesity is associated with a chronic, low-grade inflammatory state. Obesity-related inflammatory responses can be partially mediated by oxidative stress (Wood et al., 2009). Targeted intervention of inflammatory pathways may help to prevent obesity-associated metabolic disorders (Gregor and Hotamisligil, 2011). Naturally occurring antioxidant compounds including flavonoids, anthocyanidins, and catechins have been reported to affect the regulation of lipid metabolism through modulating oxidative stress conditions (Hogan et al., 2010; Yen et al., 2011).
Myricetin is a major flavonoid found in various foods, including onions, bayberries, grapes, and tea (Harnly et al., 2006; Chen W. et al., 2011; Li et al., 2012). Accumulating evidence shows that myricetin has antioxidant, anti-inflammatory, and anticancer activities (Lu et al., 2006; Chen et al., 2013a; 2013b; 2015). In addition, it can lower plasma-glucose levels, improve the insulin-signaling pathway, and reduce hyperglycemia (Ong and Khoo, 2000; Liu et al., 2005; 2007). Myricetin is also confirmed to have therapeutic benefits in relation to cardiovascular diseases associated with obesity (Ong and Khoo, 1997). However, little information is available with respect to the direct anti-obesity effects of myricetin on high-fat diet (HFD)-induced C57BL/6 mice through modulating the oxidative stress pathway.
In this study, we aimed to investigate the effects of myricetin on the development of obesity in HFD-induced C57BL/6 mice by examining serum glucose, triglyceride (TG), and cholesterol profiles as well as oxidative stress indicators. We then evaluated the gene expression of adipogenesis- and lipogenesis-related transcription factors in epididymal white adipocytes. Our results provide a foundation for future studies on the application of myricetin for the preclinical prevention and treatment of obesity.
2 Materials and methods
2.1 Reagents
Myricetin (purity >96%) was obtained from the National Institutes for Food and Drug Control (Beijing, China). Oil Red O was purchased from Sigma-Aldrich Co. (CA, USA), TRIzol from Invitrogen (Carlsbad, CA, USA), and SYBR Green PCR Master Mix from Roche (Basel, Switzerland). All other reagents used were of analytical grade.
2.2 Animal experiments
All the animal experimental procedures in this study were conducted in the Animal Experiment Center of Zhejiang University (Hangzhou, China). The animal protocol was approved by the Institutional Animal Care and Use Committee of Zhejiang University. Animal diets were obtained from Medicience Ltd. (Yangzhou, China). Myricetin was dissolved in normal saline (NS)/Tween-80 (25:1, v/v) by grinding with a mortar and pestle. Male C57BL/6 mice at 4 weeks of age were purchased from the Silaike Experimental Animal Co., Ltd. (Shanghai, China). The mice were housed in a pathogen-free facility under a 12-h light/12-h dark cycle ((22±2) °C, 40%–70% relative humidity), and had ad libitum access to water and diet. After 5-d adaptation, the mice were randomly divided into two groups: a normal diet group (ND) fed a diet containing 10% fat, and an HFD group fed a diet containing 45% fat. Both diets were maintained for 2 weeks. For the obesity challenge study: (1) 12 mice in the ND group were fed an ND administered with vehicle (NS/Tween-80 (25:1, v/v)) by oral gavage; (2) 12 mice in the HFD group were fed an HFD administered with vehicle (NS/Tween-80 (25:1, v/v)) by oral gavage; (3) another 12 mice in the HFD group were fed an HFD with myricetin at 150 mg/(kg·d) (M150), in accordance with Choi et al. (2014). All mice were permitted ad libitum consumption of water. After 10 weeks, all mice were sacrificed following 12-h fasting. Blood samples were collected, and liver and adipose tissue samples were collected, weighed, and stored at −80 °C for further assessment.
2.3 Biochemical analyses
The blood samples were centrifuged at 3000 r/min for 15 min and then were collected for biochemical analyses. Serum glucose, TG, total cholesterol (TC), and malondialdehyde (MDA) levels were measured using commercial enzyme kits (Elabscience, China) according to the manufacturer’s protocols. Glutathione peroxidase (GPX), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC) were determined by enzymatic methods using ELISA kits (Sigma, CA, USA) according to the manufacturer’s instructions (Wu et al., 2013c; Yu et al., 2016).
2.4 Hematoxylin and eosin staining
Morphological analysis of adipocytes was performed on hematoxylin and eosin (H&E)-stained sections as described (Wu et al., 2013a). Epididymal white adipose tissue samples were dissected and fixed in 10% formalin for 24 h, then dehydrated by ethanol solutions. The samples were then embedded in paraffin wax and sliced. The fixed tissue slices were stained with H&E, mounted by neutral sizing, and dried. Images were obtained using a Nikon microscope (Tokyo, Japan).
2.5 Oil Red O staining
Oil Red O staining was performed as described by Wu et al. (2013b). Sections, approximately 4–5 µm thick, were cut from liver tissue using a freeze microtome. The sections were fixed in 4% formaldehyde for 20 min and then stained with Oil Red O for 30 min. The slices were washed with 60% isopropanol three times to remove unbound dye and were then photographed. Images were obtained using a Nikon microscope.
2.6 Real-time reverse transcription-PCR
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed as described by Wu et al. (2014) and Lei et al. (2015). Total RNA was isolated from the epididymal adipose tissue of each mouse using TRIzol (Invitrogen) and then pooled for the RT-PCR analysis. Single-stranded complementary DNA (cDNA) was synthesized using the PrimeScript RT reagent kit (TaKaRa, Japan) according to the manufacturer’s instruction. Quantitative real-time PCR was carried out using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The 20-µl reaction mixture consisted of 1× SYBR Green PCR Master Mix, 0.25 µmol/L of cDNA, and 0.2 µmol/L of each primer. The PCR conditions were as follows: denaturation for 10 min at 95 °C, followed by 40 cycles at 95 °C for 10 s, and then at 60 °C for 60 s. The following primers were used: PPARγ, 5′-CGC TGA TGC ACT GCC TAT GA-3′ (forward) and 5′-AGA GGT CCA CAG AGC TGA TTC-3′ (reverse); C/EBP-α, 5′-AGC AAC GAG TAC CGG GTA CG-3′ (forward) and 5′-TGT TTG GCT TTA TCT CGG CTC-3′ (reverse); SREBP-1c, 5′-TTG TGG AGC TCA AAG ACC TG-3′ (forward) and 5′-TGC AAG AAG CGG ATG TAG TC-3′ (reverse); β-ACTIN, 5′-ATG TGG ATC AGC AAG CAG GA-3′ (forward) and 5′-AAG GGT GTA AAA CGC AGC TCA-3′ (reverse). A relative gene-expression quantification method was used to calculate the fold change in mRNA expression according to the comparative CT method using β-ACTIN for normalization. Results were obtained from at least three independent experiments.
2.7 Statistical analysis
All experimental data were analyzed using SPSS (Version 20.0) and expressed as the mean±standard deviation (SD). One-way analysis of variance (ANOVA) was used to evaluate the statistical differences among different groups. A P-value of <0.05 was considered statistically significant.
3 Results
3.1 Myricetin ameliorates obesity in HFD-induced mice
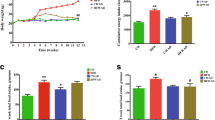
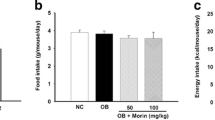
In the current study, C57BL/6 mice were fed an ND, HFD, or HFD+M (150 mg/(kg·d)) by oral gavage for 10 weeks. HFD-fed mice had a significantly higher body weight than ND-fed mice (Fig. 1a). Administration of myricetin significantly reduced the body weight of mice fed an HFD. At the end of the experiments, a 19.8% reduction in body weight was observed in the myricetin-treated group relative to the HFD-fed control group. There were no significant differences in the food or calorie intake between the HFD and HFD+M groups (Figs. 1b and 1c), suggesting that food intake did not have a significant role in the manipulation of the body weight of the mice.
Effects of myricetin on HFD-induced obese mice
Changes in body weight (a), food intake (b), calorie intake (c), epididymal fat (d), subcutaneous fat (e), perirenal fat (f), and total fat (g) by the treatment of myricetin (150 mg/(kg·d)) or vehicle for male C57BL/6 mice. Data represent mean±SD (n=12). # P<0.05 versus ND group; *P<0.05 versus HFD group. 1 kcal=4.1868 kJ
3.2 Myricetin reduces visceral fat in HFD-induced mice
To determine the effect of myricetin on visceral fat, mice adipose tissues were excised and weighed. The HFD led to significant increases in epididymal (148.2%, Fig. 1d), subcutaneous (208.7%, Fig. 1e), perirenal (205.6%, Fig. 1f), and total (170.4%, Fig. 1g) fat relative to the ND-fed group. Consistent with our expectation, administration of myricetin significantly reduced these increases, to 43.2% (epididymal), 46.5% (subcutaneous), 49.1% (perirenal), and 45.3% (total fat). In conclusion, myricetin provides protection against obesity in HFD-induced mice by decreasing visceral fat accumulation.
3.3 Myricetin reduces serum biochemical parameters in HFD-induced mice
Obesity is accepted as a chronic disease which is associated with alteration of serum parameters. Therefore, we investigated serum glucose (Fig. 2a), total TG (Fig. 2b), and cholesterol (Fig. 2c) concentrations with or without myricetin treatment. The glucose and total TG levels were significantly elevated in the HFD treatment group. However, there were no significant differences in the levels of glucose and TG between the ND group and the myricetin-treated group. Myricetin-treated mice also showed a slight reduction in serum cholesterol compared with those in the HFD-induced group.
3.4 Effects of myricetin on adiponectin, leptin, TNF-α, and insulin
We determined adiponectin, leptin, tumor necrosis factor-α (TNF-α), and insulin levels. The HFD group showed a 28.17% reduction in serum adiponectin compared with the ND group (Fig. 3a). Administration of myricetin resulted in a 15.77% increase in adiponectin compared with the HFD-treated group. The leptin level was significantly increased by 100% in HFD-fed mice relative to ND-fed mice. However, in mice receiving myricetin the increase in leptin level was reduced to 55.34% (Fig. 3b). Next, we investigated the effect of myricetin administration on the inflammation marker TNF-α in HFD-induced mice. A marked increase in the production of TNF-α was observed in HFD-fed mice compared with ND-fed mice (Fig. 3c). The myricetin treatment group showed a much smaller increase in TNF-α content. To identify the effect of myricetin on obesity-associated insulin resistance, we determined the insulin level. The HFD significantly increased the insulin level by 28.63%, whereas myricetin-treated mice had slightly less insulin than those in the ND group (Fig. 3d).
3.5 Effects of myricetin on serum antioxidant capacity
A growing amount of evidence indicates that obesity is linked to excessive oxidative stress. Therefore, we investigated the antioxidant capacity of myricetin in HFD-fed mice. GPX is an important endogenous antioxidant enzyme that is responsible for reactive oxygen species (ROS) scavenging. The serum GPX content was 24.6% lower in the HFD group than in the ND group (Fig. 4a). Administration of myricetin significantly elevated the amount of GPX by 49.6% compared with the amount in the HFD group. Our results showed a significant elevation of MDA level by 51.7% in the HFD group compared with that in the ND-treated group (Fig. 4b). However, the MDA level in the myricetin-treated group showed no significant increase, suggesting that myricetin could correct the lipid peroxidation condition. Further investigation showed that there were no significant differences in serum SOD activity among ND, HFD, and myricetin-treated groups (Fig. 4c). In addition, myricetin showed the ability to overcome the reduction in T-AOC induced by the HFD (Fig. 4d).
3.6 Effects of myricetin on morphology of white adipose and liver tissues
To characterize the morphological change in adipocytes at the end of myricetin treatment, we analyzed the epididymal white adipose tissue samples. The adipocytes in the ND group were small with clear morphology and complete structure (Fig. 5a). Adipocytes of the HFD group showed an uneven size and irregular arrangement. Strikingly, a decrease in the diameter of adipocytes of epididymal fats was noticed in mice treated with myricetin compared with those in the HFD group. Based on these observations, we conclude that myricetin can suppress fat accumulation in white adipose tissue. Next, we performed a histological analysis of liver sections from mice receiving diets with or without myricetin (Fig. 5b). The liver sections were stained by Oil Red O and then examined by microscopic imaging. The liver sections from HFD-fed mice exhibited a red color indicative of lipid accumulation. Conversely, administration of myricetin attenuated HFD-induced fat accumulation in the liver tissue.
Effects of myricetin on the fat content in adipose and liver tissues
(a) Effects of myricetin on morphological changes in epididymal adipose tissue. H&E stained epididymal adipose tissue.
(b) Effects of myricetin on lipid accumulation in liver section. Oil-Red O stained mice liver section. The samples were examined using Nikon (Tokyo, Japan) microscope imaging
3.7 Effects of myricetin on obesity-related transcription factors in epididymal white adipose tissue
The reduction in body weight of HFD-induced mice receiving myricetin suggested that myricetin was capable of regulating transcription factors involved in adipogenesis (including adipogenic genes: peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding protein α (C/EBPα)) and lipogenesis (sterol regulatory element-binding protein 1c (SREBP-1c)). The fold change in gene expression in epididymal white adipocytes was measured by RT-PCR. Marked increase in PPARγ and C/EBPα gene expressions was observed in HFD-fed mice compared with ND-fed mice (Figs. 6a and 6b). Administration of myricetin significantly reduced HFD-induced upregulation of PPARγ and C/EBPα. SREBP-1c was also markedly higher in HFD-fed mice than in ND-fed mice (Fig. 6c). Mice receiving myricetin showed reduced SREBP-1c gene expression compared with HFD-fed mice. In conclusion, our results suggest that myricetin appeared to inhibit obesity partly by down-regulation of adipogenesis- and lipogenesis-related gene expressions.
4 Discussion
The prevalence and severity of obesity are growing worldwide (Ng et al., 2014). Several Food and Drug Administration (FDA)-approved anti-obesity drugs are commercially available for use in weight control and weight reduction, but undesirable side-effects have made it difficult for obese patients to achieve long-term maintenance of weight loss (Colon-Gonzalez et al., 2012; Wong et al., 2012). Therefore, safer and efficacious agents are needed urgently to inhibit the occurrence and development of obesity on a long-term basis. Recently, several natural phytochemicals derived from fruits and vegetables have been demonstrated to suppress obesity and obesity-related metabolic syndrome (Keophiphath et al., 2009; Chen Y.K. et al., 2011; Ueda and Ashida, 2012). In the present study, we found that myricetin, a naturally occurring small molecule, could provide protection against HFD-induced obesity in C57BL/6 mice.
HFD leads to a progressive gain in body weight both in humans and animals. In this study, HFD caused elevated body weight in C57BL/6 mice, but administration of myricetin attenuated the HFD-induced weight gain. Given that there were no significant differences in food and calorie intake between the HFD and HFD+M groups, we conclude that the appetites of the mice were unaffected by myricetin treatment. Myricetin administration also attenuated increases in glucose, total TG, and TC levels in the serum caused by HFD. Leptin is an adipocyte-derived hormone that contributes to maintenance of food intake and improvement of energy homeostasis in peripheral tissues, such as the liver and muscle (Kim and Park, 2011). After HFD treatment, leptin levels increased notably in our experiment, in accordance with a previous report (Frederich et al., 1995). Adiponectin, produced by adipose tissue, has been shown to stimulate food intake and decrease energy expenditure (Kubota et al., 2007). We found that administration of myricetin increased the levels of leptin and decreased the levels of adiponectin in obese mice, suggesting that adiponectin and leptin are involved in the antiobesity action of myricetin.
Excessive oxidative stress has been characterized in obesity and obesity-related metabolic complications. Accumulating evidence suggests that antioxidant defense systems are impaired in diet-induced obesity (Hogan et al., 2010; Yen et al., 2011). GPXs play a pivotal role in the maintenance of physiologically produced ROS. MDA, as an important lipid peroxidation product, has been used as a marker for evaluation of oxidative stress. In the present study, we found myricetin significantly promoted GPX activity and decreased MDA levels in HFD-fed mice, suggesting that myricetin could improve antioxidant capacity in vivo. Oxidative stress is closely linked to inflammation in obesity (Wood et al., 2009) and elevated pro-inflammatory cytokines have been reported to augment the development of the metabolic syndrome associated with obesity (Hotamisligil et al., 1996). TNF-α is a pro-inflammatory cytokine that has a critical role in multiple inflammatory diseases, including obesity, diabetes, osteoporosis, and rheumatoid arthritis (Feldmann and Maini, 2001; Xu et al., 2002; Tweedie et al., 2007). We found that administration of myricetin reduced TNF-α levels in HFD-induced mice. Collectively, these results suggest that the development of obesity and obesity-related metabolic syndrome could be prevented by myricetin through modulating oxidative stress conditions.
Adipose tissue grows by hypertrophy (cell size increase) and hyperplasia (cell number increase) in obese mice. Lipid is stored in adipose tissues when energy intake exceeds energy expenditure, resulting in hypertrophy and body weight gain (Kim and Park, 2011). Myricetin treatment effectively reduced the size and number of epididymal adipocytes and hepatocytes, and lessened fat mass compared with the HFD-induced mice without myricetin. This observation suggests that myricetin might inhibit the process of adipogenesis and lipid accumulation during cell differentiation. To gain more mechanistic insight into the anti-obesity effect of myricetin, we examined the expression of lipogenic- and adipogenic-related genes. PPARγ and C/EBPα are regulators of many genes involved in the adipose phenotype and lipid metabolism (Hou et al., 2012). Administration of myricetin resulted in a down-regulation of PPARγ and C/EBPα. SREBP-1c regulates the expression of target genes involved in fatty acid synthesis (Joseph et al., 2002). The pronounced decrease in serum and hepatic TG found in the myricetin-treated group suggests that altered gene expression of SREBP-1c in adipocytes might be an underlying mechanism. This hypothesis was confirmed by the observation that the expression of lipogenic transcription factor SREBP-1c was suppressed by myricetin treatment. Taken together, these observations suggest that myricetin may suppress the expression of adipogenic and lipogenic genes, thereby regulating the onset of obesity.
5 Conclusions
In summary, the present study showed that myricetin decreased serum glucose levels, regulated lipid metabolism, and provided protection against HFD-induced oxidative stress in obese mice. A further mechanistic study revealed that administration of myricetin down-regulated the expression of adipogenic (PPARγ and C/EBPα) and lipogenic (SREBP-1c) genes in HFD-induced mice. No deaths or abnormalities resulted from this experiment. Therefore, myricetin may be a safe and effective agent for preventing obesity. However, there is no direct evidence showing that myricetin has the same anti-obesity capability in humans and therefore future clinical studies need to be considered.
Compliance with ethics guidelines
Hong-ming SU, Li-na FENG, Xiao-dong ZHENG, and Wei CHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
Chen, W., Li, Y., Li, J., et al., 2011. Myricetin affords protection against peroxynitrite-mediated DNA damage and hydroxyl radical formation. Food Chem. Toxicol., 49(9):2439–2444. http://dx.doi.org/10.1016/j.fct.2011.06.066
Chen, W., Feng, L., Shen, Y., et al., 2013a. Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. Biomed. Res. Int., 2013:724183. http://dx.doi.org/10.1155/2013/724183
Chen, W., Zhuang, J., Li, Y., et al., 2013b. Myricitrin protects against peroxynitrite-mediated DNA damage and cytotoxicity in astrocytes. Food Chem., 141(2):927–933. http://dx.doi.org/10.1016/j.foodchem.2013.04.033
Chen, W., Zhou, S., Zheng, X., 2015. A new function of Chinese bayberry extract: protection against oxidative DNA damage. LWT-Food Sci. Technol., 60(2):1200–1205. http://dx.doi.org/10.1016/j.lwt.2014.09.011
Chen, Y.K., Cheung, C., Reuhl, K.R., et al., 2011. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem., 59(21):11862–11871. http://dx.doi.org/10.1021/jf2029016
Choi, H.N., Kang, M.J., Lee, S.J., et al., 2014. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr. Res. Pract., 8(5):544–549. http://dx.doi.org/10.4162/nrp.2014.8.5.544
Colon-Gonzalez, F., Kim, G.W., Lin, J.E., et al., 2012. Obesity pharmacotherapy: what is next? Mol. Aspects Med., 34(1):71–83. http://dx.doi.org/10.1016/j.mam.2012.10.005
Feldmann, M., Maini, R.N., 2001. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol., 19(1):163–196. http://dx.doi.org/10.1146/annurev.immunol.19.1.163
Frederich, R.C., Hamann, A., Anderson, S., et al., 1995. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med., 1(12):1311–1314. http://dx.doi.org/10.1038/nm1295-1311
Furukawa, S., Fujita, T., Shimabukuro, M., et al., 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest., 114(12):1752–1761. http://dx.doi.org/10.1172/JCI21625
Galinier, A., Carriere, A., Fernandez, Y., et al., 2006. Adipose tissue proadipogenic redox changes in obesity. J. Biol. Chem., 281(18):12682–12687. http://dx.doi.org/10.1074/jbc.M506949200
Gregor, M.F., Hotamisligil, G.S., 2011. Inflammatory mechanisms in obesity. Annu. Rev. Immunol., 29(1):415–445. http://dx.doi.org/10.1146/annurev-immunol-031210-101322
Grundy, S.M., 1998. Multifactorial causation of obesity: implications for prevention. Am. J. Clin. Nutr., 67(Suppl. 3): 563S–572S.
Harnly, J.M., Doherty, R.F., Beecher, G.R., et al., 2006. Flavonoid content of us fruits, vegetables, and nuts. J. Agric. Food Chem., 54(26):9966–9977. http://dx.doi.org/10.1021/jf061478a
Hogan, S., Canning, C., Sun, S., et al., 2010. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food Chem., 58(21):11250–11256. http://dx.doi.org/10.1021/jf102759e
Hotamisligil, G.S., Peraldi, P., Budavari, A., et al., 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science, 271(5249):665–670. http://dx.doi.org/10.1126/science.271.5249.665
Hou, Y., Xue, P., Bai, Y., et al., 2012. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein β during adipogenesis. Free Radic. Biol. Med., 52(2):462–472. http://dx.doi.org/10.1016/j.freeradbiomed.2011.10.453
Jeong, M.Y., Kim, H.L., Park, J., et al., 2014. Rubi Fructus (Rubus coreanus) activates the expression of thermogenic genes in vivo and in vitro. Int. J. Obes., 39(3):456–464. http://dx.doi.org/10.1038/ijo.2014.155
Joseph, S.B., Laffitte, B.A., Patel, P.H., et al., 2002. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem., 277(13):11019–11025. http://dx.doi.org/10.1074/jbc.M111041200
Keophiphath, M., Priem, F., Jacquemond-Collet, I., et al., 2009. 1,2-Vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J. Nutr., 139(11):2055–2060. http://dx.doi.org/10.3945/jn.109.105452
Kim, K.H., Park, Y., 2011. Food components with anti-obesity effect. Annu. Rev. Food Sci. Technol., 2(1):237–257. http://dx.doi.org/10.1146/annurev-food-022510-133656
Kubota, N., Yano, W., Kubota, T., et al., 2007. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab., 6(1):55–68. http://dx.doi.org/10.1016/j.cmet.2007.06.003
Lei, K., Li, Y.L., Wang, Y., et al., 2015. Effect of dietary supplementation of Bacillus subtilis B10 on biochemical and molecular parameters in the serum and liver of high-fat diet-induced obese mice. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 16(6):487–495. http://dx.doi.org/10.1631/jzus.B1400342
Li, J., Han, Q., Chen, W., et al., 2012. Antimicrobial activity of Chinese bayberry extract for the preservation of surimi. J. Sci. Food Agric., 92(11):2358–2365. http://dx.doi.org/10.1002/jsfa.5641
Liu, I.M., Liou, S.S., Lan, T.W., et al., 2005. Myricetin as the active principle of abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med., 71(7):617–621. http://dx.doi.org/10.1055/s-2005-871266
Liu, I.M., Tzeng, T.F., Liou, S.S., et al., 2007. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci., 81(21):1479–1488. http://dx.doi.org/10.1016/j.lfs.2007.08.045
Lu, J., Papp, L.V., Fang, J., et al., 2006. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Res., 66(8):4410–4418. http://dx.doi.org/10.1158/0008-5472.CAN-05-3310
Ng, M., Fleming, T., Robinson, M., et al., 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet, 384(9945):766–781. http://dx.doi.org/10.1016/s0140-6736(14)60460-8
Ong, K.C., Khoo, H.E., 1997. Biological effects of myricetin. Gen. Pharmacol. Vasc. Syst., 29(2):121–126. http://dx.doi.org/10.1016/S0306-3623(96)00421-1
Ong, K.C., Khoo, H.E., 2000. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci., 67(14):1695–1705. http://dx.doi.org/10.1016/S0024-3205(00)00758-X
Song, Y., Park, H.J., Kang, S.N., et al., 2013. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS ONE, 8(7):e69925. http://dx.doi.org/10.1371/journal.pone.0069925
Tilg, H., Moschen, A.R., 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol., 6(10):772–783. http://dx.doi.org/10.1038/nri1937
Tweedie, D., Sambamurti, K., Greig, N.H., 2007. TNF-α inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr. Alzheimer Res., 4(4):378–385. http://dx.doi.org/10.2174/156720507781788873
Ueda, M., Ashida, H., 2012. Green tea prevents obesity by increasing expression of insulin-like growth factor binding protein-1 in adipose tissue of high-fat diet-fed mice. J. Agric. Food Chem., 60(36):8917–8923. http://dx.doi.org/10.1021/jf2053788
Wong, D., Sullivan, K., Heap, G., 2012. The pharmaceutical market for obesity therapies. Nat. Rev. Drug Discov., 11(9):669–670. http://dx.doi.org/10.1038/nrd3830
Wood, I.S., de Heredia, F.P., Wang, B., et al., 2009. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc. Nutr. Soc., 68(4):370–377. http://dx.doi.org/10.1017/S0029665109990206
Wu, T., Tang, Q., Gao, Z., et al., 2013a. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE, 8(10):e77585. http://dx.doi.org/10.1371/journal.pone.0077585
Wu, T., Qi, X., Liu, Y., et al., 2013b. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem., 141(1):482–487. http://dx.doi.org/10.1016/j.foodchem.2013.03.046
Wu, T., Yu, Z., Tang, Q., et al., 2013c. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct., 4(11):1654–1661. http://dx.doi.org/10.1039/c3fo60251f
Wu, T., Tang, Q., Yu, Z., et al., 2014. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int. J. Food Sci. Nutr., 65(3):351–359. http://dx.doi.org/10.3109/09637486.2013.854749
Xu, H., Uysal, K.T., Becherer, J.D., et al., 2002. Altered tumor necrosis factor-α (TNF-α) processing in adipocytes and increased expression of transmembrane TNF-α in obesity. Diabetes, 51(6):1876–1883. http://dx.doi.org/10.2337/diabetes.51.6.1876
Yen, G.C., Chen, Y.C., Chang, W.T., et al., 2011. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J. Agric. Food Chem., 59(2):546–551. http://dx.doi.org/10.1021/jf1036992
Yu, Z.P., Xu, D.D., Lu, L.F., et al., 2016. Immunomodulatory effect of a formula developed from American ginseng and Chinese jujube extracts in mice. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 17(2):147–157. http://dx.doi.org/10.1631/jzus.B1500170
Yun, J.W., 2010. Possible anti-obesity therapeutics from nature—a review. Phytochemistry, 71(14–15):1625–1641. http://dx.doi.org/10.1016/j.phytochem.2010.07.011
Zhao, Y., Sedighi, R., Wang, P., et al., 2015. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem., 63(19):4843–4852. http://dx.doi.org/10.1021/acs.jafc.5b01246
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Scientific Research Foundation of the Education Department of Zhejiang Province (No. Y201328143), the Food Science and Engineering the Most Important Discipline of Zhejiang Province (No. JYTSP20142012), the National Key Technology R & D Program of China (No. 2012BAD33B08), and the Foundation of Fuli Institute of Food Science, Zhejiang University (2013), China
ORCID: Wei CHEN, http://orcid.org/0000-0002-2373-2437
Rights and permissions
About this article
Cite this article
Su, Hm., Feng, Ln., Zheng, Xd. et al. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. J. Zhejiang Univ. Sci. B 17, 437–446 (2016). https://doi.org/10.1631/jzus.B1600074
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1600074