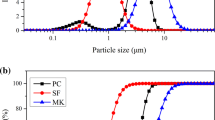
Abstract
Solid–liquid equilibrium curve of calcium (Ca) is important for analyzing the process of calcium leaching and its kinetics model. In this paper, the Ca solid–liquid equilibrium was experimentally investigated by hardened cement paste powder exposed to an accelerated leaching environment of 1 mol/L (1 M), 3 M and 6 M ammonium chloride (NH4Cl) solutions. The equilibrium curves of Ca, magnesium (Mg), sulfur (S), aluminum (Al) and iron (Fe) were obtained by EDTA titration and scanning electron microscope-electronic diffraction spectroscopy. Results show that, the obtained equilibrium curve in NH4Cl solution has a similar three-stage form to that in deionized water. It indicates that, the Ca leaching mechanism of cement based materials in ammonium solution is similar to that in water. In addition, the Ca leaching in NH4Cl solution is accompanied by the leaching of S and Mg, and they are also leached in three stages, but Al and Fe are not leachable. Based on the experimental data, the equations of Ca solid–liquid equilibrium curve under 1 M, 3 M and 6 M NH4Cl solutions were established.









Similar content being viewed by others
References
Sun W (2015) Durability evaluation and service life prediction of modern concrete. China Architecture & Building Press, Beijing, China.
Jin W, Zhao X (2014) Durability of concrete structures. Science Press, Beijing, China
Jin W, Yuan Y, Wei J, et al (2011) Durability theory and design method of concrete structure in chloride environment. Science Press, Beijing, China.
Yang H, Che Y, Leng F (2018) Calcium leaching behavior of cementitious materials in hydrochloric acid solution. Sci Rep 8:1–10
Liu L, Wang X, Zhou J et al (2018) Investigation of pore structure and mechanical property of cement paste subjected to the coupled action of freezing/thawing and calcium leaching. Cem Concr Res 109:133–146
Weiting L, An C, Ran H et al (2016) Effect of calcium leaching on the properties of cement-based composites. J Wuhan Univ Technol 26:990–997
Sarkar S, Mahadevan S, Meeussen JCL et al (2012) Sensitivity analysis of damage in cement materials under sulfate attack and calcium leaching. J Mater Civ Eng 24:430–440
Rozi Re E, Loukili A (2011) Performance-based assessment of concrete resistance to leaching. Cement Concr Compos 33:451–456
Ryu J, Otsuki N, Minagawa H (2002) Long-term forecast of Ca leaching from mortar and associated degeneration. Cement Concr Res 32:1539–1544
Alonso C, Castellote M et al (2006) Ground water leaching resistance of high and ultra-high performance concretes in relation to the testing convection regime. Cem Concr Res 36:1583–1594
Mainguy M, Tognazzi C, Torrenti JM et al (2000) Modelling of leaching in pure cement paste and mortar. Cem Concr Res 30:83–90
Glasser FP, Marchand J, Samson E (2008) Durability of concrete—Degradation phenomena involving detrimental chemical reactions. Cem Concr Res 38:226–246
Kamali S (2003) Comportement et simulation des matériaux cimentaires en environnement agressifs: Lixiviation et température. Bibliogr
Zhang L (2007) Research on the electrochemical approach to accelerate the dissolution of concrete. Hohai University
Saito H, Deguchi A (2000) Leaching tests on different mortars using accelerated electrochemical method. Cem Concr Res 30:1815–1825
Le Chatelier H (1905) Experimental researches on the constitution of hydraulic mortars. J Am Chem Soc 8:1028–1029
Haga K, Sutou S, Hironaga M et al (2005) Effects of porosity on leaching of Ca from hardened ordinary Portland cement paste. Cem Concr Res 35:1764–1775
Mainguy M, Coussy O (2016) Propagation fronts during calcium leaching and chloride penetration. J Eng Mech 1:250–257
Kamali S, Moranville M, Leclercq SP (2008) Material and environmental parameter effects on the leaching of cement pastes: experiments and modelling. Cem Concr Res 38:575–585
Bertron A, Duchesne J, Escadeillas G (2005) Accelerated tests of hardened cement pastes alteration by organic acids: analysis of the pH effect. Cem Concr Res 35:155–166
Hidalgo A, Petit S, Domingo C et al (2007) Microstructural characterization of leaching effects in cement pastes due to neutralisation of their alkaline nature. Cem Concr Res 37:63–70
Pichler C, Saxer A, Lackner R (2014) Differential-scheme based dissolution/diffusion model for calcium leaching in cement-based materials accounting for mix design and binder composition. Cem Concr Res 58:201–203
Ulm FJ, Lemarchand E, Heukamp FH (2003) Elements of chemomechanics of calcium leaching of cement-based materials at different scales. Eng Fract Mech 70:871–889
Nguyen VH, Nedjar B et al (2006) A separation of scales homogenization analysis for the modelling of calcium leaching in concrete. Comput Methods Appl Mech Eng 195:7196–7210
Burlion N, Bernard D, Chen D (2006) X-ray microtomography: application to microstructure analysis of a cementitious material during leaching process. Cem Concr Res 36:346–357
Nakarai K, Ishida T, Maekawa K (2006) Modeling of calcium leaching from cement hydrates coupled with micro-pore formation. J Adv Concr Technol 4:395–407
Tang Y, Zuo X, He S et al (2016) Influence of slag content and water-binder ratio on leaching behavior of cement pastes. Constr Build Mater 129:61–69
Zuo X, Tang Y et al (2017) Influence of fly ash and its partial replacement by slag on the leaching behavior of blended cement pastes. J Mater Civ Eng 29:1–11
Kaiqian X, Ying Z et al (2020) Summary of experimental methods for accelerated concrete corrosion. Concrete 3:13–19
Carde C, Escadeillas G, Francois R (1997) Use of ammonium nitrate solution to simulate and accelerate the leaching of cement pastes due to deionized water. Mag Concr Res 181:295–301
Thomas JJ, Chen JJ, Allen AJ et al (2004) Effects of decalcification on the microstructure and surface area of cement and tricalcium silicate pastes. Cement Concr Res 34:2297–2307
Tang Y-J, Zuo X-B et al (2017) Influence of slag on leaching behavior of cement mortar lined in ductile iron pipe under a flowing solution. Mater Des 114:612–522
Xie SY, Shao JF, Burlion N (2008) Experimental study of mechanical behaviour of cement paste under compressive stress and chemical degradation. Cem Concr Res 38:1416–1423
Pichler C, Saxer A, Unterberger S et al (2014) Reply to discussion of paper “differential-scheme based dissolution/diffusion model for calcium leaching in cement-based materials accounting for mix design and binder composition.” Cem Concr Res 58:99–102
Forster AM, Szadurski EM, Banfill PFG (2014) Deterioration of natural hydraulic lime mortars, I: effects of chemically accelerated leaching on physical and mechanical properties of uncarbonated materials. Constr Build Mater 72:199–207
Laws and regulations (2006) Regulations on safety management of civil explosives. Beijing, China
Hu Y, Jiang L, Yan Z, Qi P, Yi X (2012) Predicting the calcium leaching behavior of cement pastes in aggressive environments. Constr Build Mater 29:88–96
Buil M (1991) A model of the attack of pure water or under saturated lime solution on cement. Astm Spt 1123:227–241
Greenberg SA, Chang TN (1965) Investigation of the colloidal hydrated calcium silicates. II. Solubility relationships in the calcium oxide-silica-water system at 25°. J Phys Chem 69:182–188
Chen JJ, Thomas JJ, Taylor HFW et al (2004) Solubility and structure of calcium silicate hydrate. Cem Concr Res 34:1499–1519
Damidot D, Lothenbach B, Herfort D et al (2011) Thermodynamics and cement science. Cem Concr Res 41:679–695
Li L (2014) The research of calcium leaching of hardened cement pastes. Southeast University, Dhaka
Jain J, Neithalath N (2009) Analysis of calcium leaching behavior of plain and modified cement pastes in pure water. Cement Concr Compos 31:176–185
Jennings H (1986) Aqueous solubility relationships for two types of calcium silicate hydrate. J Am Ceram Soc 69:614–618
Wan K, Li Y, Sun W (2013) Experimental and modelling research of the accelerated calcium leaching of cement paste in ammonium nitrate solution. Constr Build Mater 40:832–846
Bejaoui S, Bary B (2007) Modeling of the link between microstructure and effective diffusivity of cement pastes using a simplified composite model. Cem Concr Res 37:469–480
Gérard B, Le Bellego C, Bernard O (2002) Simplified modelling of calcium leaching of concrete in various environments. Mater Struct 35:632–640
Heukamp FH, Ulm FJ, Germaine JT (2001) Mechanical properties of calcium-leached cement pastes triaxial stress states and the influence of the pore pressures. Cem Concr Res 31:767–774
Berner UR (1988) Modelling the incongruent dissolution of hydrated cement minerals. Radiochim Acta 44:387–394
Buil M (1992) A model of the attack of pure water or undersaturated lime solutions on cement. ASTM STP 1123:227–241
Wan K, Li L, Sun W (2013) Solid-liquid equilibrium curve of calcium in 6 mol/L ammonium nitrate solution. Cem Concr Res 53:44–50
Shaowei W, Yanyu X et al (2022) Acceleration effect of chemical solution immersion on calcium leaching of cement-based materials. J Chin Ceram Soc 50:403–412
Chen JJ, Thomas JJ, Jennings HM (2006) Decalcification shrinkage of cement paste. Cem Concr Res 36:801–809
Puertas F, Go IS, Hern Ndez MS et al (2012) Comparative study of accelerated decalcification process among C3S, grey and white cement pastes. Cement Concr Compos 34:384–391
Buil M (1992) A model of the attack of pure water or under saturated lime solutions on cement. ASTM STP 1123:227–241
Wan KS, Li Y, Sun W (2013) Experimental and modeling research of the accelerated calcium leaching of cement paste in ammonium nitrate solution. Constr Build Mater 40:832–846
Hou Y (2012) Cementitious materials. Beijing, China
Aldridge LP (1982) Accuracy and precision of phase analysis in portland cement by Bogue, microscopic and X-ray diffraction methods. Cem Concr Res 12:381–398
Acknowledgements
The study of this paper is financially supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB430030), Zhejiang Provincial Natural Science Foundation of China (LQ21E080008), Yangzhou “Lv-yang-jin-feng” project of China, Jiangsu innovation training program for college students of China (202111462014Y) and by Scientific Research Start-up Foundation of Ningbo University of Technology in 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, Y., Yin, G., Chen, F. et al. Solid–liquid equilibrium state and equation of cement-based materials in ammonium chloride solution. Mater Struct 55, 220 (2022). https://doi.org/10.1617/s11527-022-02062-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-022-02062-z