Abstract
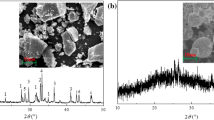
The effects of magnesium content in the precursors on the phase formation and microstructural variation of alkali-activated binders under hydrothermal conditions are investigated. One-step hydrothermal treatment method was applied on all samples synthesized from industrial by-products (e.g., fly ash and slag), MgO powders, and alkali activator, and they were characterized by compressive strength tests, XRD, SEM, N2 sorption, and FTIR analyses. The samples containing zeolite Na-P1 and hydrotalcite crystals were obtained until the Mg/(Al + Si) molar ratio was higher than 0.58 with a 20 wt.% slag content in the precursor, however, the samples with 40 wt.% slag content possessed both of these phases even at lower Mg/(Al + Si) molar ratio of 0.39. Specifically, increasing the Mg/(Al + Si) ratio significantly reduced the formation of zeolite Na-P1, while promoted the formation of hydrotalcite. Furthermore, the extended hydrothermal treatment promoted the formation of zeolite Na-P1, but simultaneously reduced the formation of hydrotalcite. All samples exhibited mesoporous characteristics having major sorption behaviors of multilayer physisorption and capillary condensation.








Similar content being viewed by others
References
Provis JL (2018) Alkali-activated materials. Cem Concr Res 114:40–48. https://doi.org/10.1016/j.cemconres.2017.02.009
White CE, Provis JL, Bloomer B, Henson NJ, Page K (2013) In situ X-ray pair distribution function analysis of geopolymer gel nanostructure formation kinetics. Phys Chem Chem Phys 15:8573–8582. https://doi.org/10.1039/c3cp44342f
Walkley B, San Nicolas R, Sani MA, Gehman JD, Van Deventer JSJ, Provis JL (2016) Phase evolution of Na2O–Al2O3–iO2–H2O gels in synthetic aluminosilicate binders. Dalt Trans 45:5521–5535. https://doi.org/10.1039/c5dt04878h
Myers RJ, Bernal SA, Provis JL (2017) Phase diagrams for alkali-activated slag binders. Cem Concr Res 95:30–38. https://doi.org/10.1016/j.cemconres.2017.02.006
Garcia-Lodeiro I, Palomo A, Fernández-Jiménez A, Macphee DE (2011) Compatibility studies between N–A–S–H and C–A–S–H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem Concr Res 41:923–931. https://doi.org/10.1016/j.cemconres.2011.05.006
Rożek P, Król M, Mozgawa W (2019) Geopolymer-zeolite composites: a review. J Clean Prod 230:557–579. https://doi.org/10.1016/j.jclepro.2019.05.152
Provis JL, van Deventer JSJ (2014) Alkali activated materials: state-of-the-art report. Springer, Berlin
Bernal SA, San Nicolas R, Myers RJ, Mejía De Gutiérrez R, Puertas F, Van Deventer JSJ, Provis JL (2014) MgO content of slag controls phase evolution and structural changes induced by accelerated carbonation in alkali-activated binders. Cem Concr Res 57:33–43. https://doi.org/10.1016/j.cemconres.2013.12.003
Ismail I, Bernal SA, Provis JL, San Nicolas R, Hamdan S, Van Deventer JSJ (2014) Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem Concr Compos 45:125–135. https://doi.org/10.1016/j.cemconcomp.2013.09.006
Richardson IG, Brough AR, Groves GW, Dobson CM (1994) The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C–S–H) phase. Cem Concr Res 24:813–829. https://doi.org/10.1016/0008-8846(94)90002-7
Fernandez-Jiménez A, Puertas F (2003) Structure of calcium silicate hydrates formed in alkaline-activated slag: influence of the type of alkaline activator. J Am Ceram Soc 89:1389–1394
Garcia-Lodeiro I, Palomo A, Fernández-Jiménez A (2014) An overview of the chemistry of alkali-activated cement-based binders. Woodhead Publishing Limited. https://doi.org/10.1533/9781782422884.1.19
Walkley B, San Nicolas R, Sani MA, Bernal SA, van Deventer JSJ, Provis JL (2017) Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem Concr Res 99:155–171. https://doi.org/10.1016/j.cemconres.2017.05.006
Park SM, Jang JG, Lee HK (2018) Unlocking the role of MgO in the carbonation of alkali-activated slag cement. Inorg Chem Front 5:1661–1670. https://doi.org/10.1039/c7qi00754j
Walkley B, Nicolas RS, Bernal SA, Provis JL, Sani M (2015) The role of MgO content in the phase assemblage of alkali-activated synthetic precursors within the system CaO–MgO–Al2O3–SiO2. In: 14th Int. Congr. Chem. Cem. (2015). http://www.iccc2015beijing.org/dct/page/1.
Walkley B, San Nicolas R, Bernal S (2015) Effect of MgO incorporation on the structure of synthetic alkali-activated calcium aluminosilicate binders. In: Conf. 27th Bienn. Natl. Conf. Concr. Inst. Aust. https://www.researchgate.net/publication/303968983_Effect_of_MgO_incorporation_on_the_structure_of_synthetic_alkali-activated_calcium_aluminosilicate_binders.
Takeda H, Hashimoto S, Yokoyama H, Honda S, Iwamoto Y (2013) Characterization of zeolite in zeolite-geopolymer hybrid bulk materials derived from kaolinitic clays. Materials (Basel) 6:1767–1778. https://doi.org/10.3390/ma6051767
Zhang J, He Y, Wang YP, Mao J, Cui XM (2014) Synthesis of a self-Supporting faujasite zeolite membrane using geopolymer gel for separation of alcohol/water mixture. Mater Lett 116:167–170. https://doi.org/10.1016/j.matlet.2013.11.008
Lee NK, Khalid HR, Lee HK (2016) Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater 229:22–30. https://doi.org/10.1016/j.micromeso.2016.04.016
Qiu X, Liu Y, Li D, Yan C (2015) Preparation of NaP zeolite block from fly ash-based geopolymer via in situ hydrothermal method. J Porous Mater 22:291–299. https://doi.org/10.1007/s10934-014-9895-3
Khalid HR, Lee NK, Park SM, Abbas N, Lee HK (2018) Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J Hazard Mater 353:522–533. https://doi.org/10.1016/j.jhazmat.2018.04.049
Khalid HR, Lee NK, Choudhry I, Wang Z, Lee HK (2019) Evolution of zeolite crystals in geopolymer-supported zeolites: effects of composition of starting materials. Mater Lett. https://doi.org/10.1016/j.matlet.2018.12.044
Lee NK, Khalid HR, Lee HK (2017) Adsorption characteristics of cesium onto mesoporous geopolymers containing nano-crystalline zeolites. Microporous Mesoporous Mater 242:238–244. https://doi.org/10.1016/j.micromeso.2017.01.030
Ok YS, Yang JE, Zhang YS, Kim SJ, Chung DY (2007) Heavy metal adsorption by a formulated zeolite-Portland cement mixture. J Hazard Mater 147:91–96. https://doi.org/10.1016/j.jhazmat.2006.12.046
Singh NB, Nagpal G, Agrawal S (2018) Rachna, Water purification by using Adsorbents: A Review. Environ Technol Innov 11:187–240. https://doi.org/10.1016/j.eti.2018.05.006
Buchwald A, Hilbig H, Kaps C (2007) Alkali-activated metakaolin-slag blends - Performance and structure in dependence of their composition. J Mater Sci 42:3024–3032. https://doi.org/10.1007/s10853-006-0525-6
Ahmed IM, Gasser MS (2012) Adsorption study of anionic reactive dye from aqueous solution to Mg-Fe-CO3 layered double hydroxide (LDH). Appl Surf Sci 259:650–656. https://doi.org/10.1016/j.apsusc.2012.07.092
Bae SJ, Park S, Lee HK (2020) Role of Al in the crystal growth of alkali-activated fly ash and slag under a hydrothermal condition. Constr Build Mater 239:117842. https://doi.org/10.1016/j.conbuildmat.2019.117842
Chi M, Huang R (2013) Binding mechanism and properties of alkali-activated fly ash/slag mortars. Build. Mater Constr. https://doi.org/10.1016/j.conbuildmat.2012.11.003
He Y, Cui XM, Mao J, Liu LP, Liu XD, Chen JY (2012) The hydrothermal transformation of solid geopolymers into zeolites. Microporous Mesoporous Mater 161:187–192. https://doi.org/10.1016/j.micromeso.2012.05.039
Park S, Park HM, Yoon HN, Seo J, Yang C-M, Provis JL, Yang B (2020) Hydration kinetics and products of MgO-activated blast furnace slag. Constr Build Mater 249:118700. https://doi.org/10.1016/j.conbuildmat.2020.118700
Sharma SK, Kushwaha PK, Srivastava VK, Bhatt SD, Jasra RV (2007) Effect of hydrothermal conditions on structural and textural properties of synthetic hydrotalcites of varying Mg/Al ratio. Ind Eng Chem Res 46:4856–4865. https://doi.org/10.1021/ie061438w
Park SM, Khalid HR, Seo JH, Yoon HN, Son HM, Kim SH, Lee NK, Lee HK, Jang JG (2018) Pressure-induced geopolymerization in alkali-activated fly ash. Sustainability. 10:3538. https://doi.org/10.3390/su10103538
Puertas F, Amat T, Fernández-Jiménez A, Vázquez T (2003) Mechanical and durable behaviour of alkaline cement mortars reinforced with polypropylene fibres. Cem Concr Res 33:2031–2036. https://doi.org/10.1016/S0008-8846(03)00222-9
Jin F, Kai G, Al-Tabbaa A (2014) Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr Build Mater 51:395–404. https://doi.org/10.1016/j.conbuildmat.2015.01.082
Hwang CL, Vo DH, Tran VA, Yehualaw MD (2018) Effect of high MgO content on the performance of alkali-activated fine slag under water and air curing conditions. Constr Build Mater 186:503–513. https://doi.org/10.1016/j.conbuildmat.2018.07.129
Nath P, Sarker PK (2014) Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr Build Mater 66:163–171. https://doi.org/10.1016/j.conbuildmat.2014.05.080
Kumar S, Kumar R, Mehrotra SP (2010) Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J Mater Sci 45:607–615. https://doi.org/10.1007/s10853-009-3934-5
Lloyd NA, Rangan BV (2010) Geopolymer concrete with fly ash. 2nd Int Conf Sustain Constr Mater Technol 7:1493–1504
Hardjito D, Rangan BV (2005) Development and properties of low-calcium fly ash-based geopolymer concrete. Res Rep GC 94.
Yang KH, Cho AR, Song JK (2012) Effect of water-binder ratio on the mechanical properties of calcium hydroxide-based alkali-activated slag concrete. Constr Build Mater 29:504–511. https://doi.org/10.1016/j.conbuildmat.2011.10.062
De Silva P, Sagoe-Crenstil K (2008) Medium-term phase stability of Na2O-Al2O3-SiO2-H2O geopolymer systems. Cem Concr Res 38:870–876. https://doi.org/10.1016/j.cemconres.2007.10.003
Abdel-Gawwad HA, Abd El-Aleem S (2015) Effect of reactive magnesium oxide on properties of alkali activated slag geopolymer cement pastes. Ceram Silikaty 59:37–47
Ben Haha M, Lothenbach B, Le Saout G, Winnefeld F (2011) Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: effect of MgO. Cem Concr Res 41:955–963. https://doi.org/10.1016/j.cemconres.2011.05.002
Park SM, Jang JG, Lee NK, Lee HK (2016) Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem Concr Res 89:72–79. https://doi.org/10.1016/j.cemconres.2016.08.004
Panias D, Giannopoulou IP, Perraki T (2007) Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surfaces A Physicochem Eng Asp 301:246–254. https://doi.org/10.1016/j.colsurfa.2006.12.064
Kuwahara Y, Ohmichi T, Kamegawa T, Mori K, Yamashita H (2010) A novel conversion process for waste slag: synthesis of a hydrotalcite-like compound and zeolite from blast furnace slag and evaluation of adsorption capacities. J Mater Chem 20:5052. https://doi.org/10.1039/c0jm00518e
Wajima T, Shimizu T, Ikegami Y (2008) Zeolite synthesis from paper sludge ash with addition of diatomite. J Chem Technol Biotechnol 83:921–927. https://doi.org/10.1002/jctb.1893
Grutzeck M, Kwan S, DiCola M (2004) Zeolite formation in alkali-activated cementitious systems. Cem Concr Res 34:949–955. https://doi.org/10.1016/j.cemconres.2003.11.003
Bernal SA, Provis JL, Rose V, Mejia De Gutierrez R (2011) Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem Concr Compos 33:46–54. https://doi.org/10.1016/j.cemconcomp.2010.09.004
Jin F, Gu K, Al-Tabbaa A (2014) Strength and drying shrinkage of reactive MgO modified alkali-activated slag paste. Constr Build Mater 51:395–404. https://doi.org/10.1016/j.conbuildmat.2013.10.081
Ben Haha M, Lothenbach B, Le Saout G, Winnefeld F (2012) Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem Concr Res 42:74–83. https://doi.org/10.1016/j.cemconres.2011.08.005
Lee NK, Jang JG, Lee HK (2014) Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem Concr Compos 53:239–248. https://doi.org/10.1016/j.cemconcomp.2014.07.007
Ferrarini SF, Cardoso AM, Alban L, Pires MJR (2018) Evaluation of the sustainability of integrated hydrothermal synthesis of zeolites obtained from Waste. J Braz Chem Soc 29:1464–1479. https://doi.org/10.21577/0103-5053.20180017
Liao L, Zhao N, Xia Z (2012) Hydrothermal synthesis of Mg–Al layered double hydroxides (LDHs) from natural brucite and Al(OH)3. Mater Res Bull 47:3897–3901. https://doi.org/10.1016/j.materresbull.2012.07.007
Liu B, Yang J, Li D, Xing F, Fang Y (2018) Effect of a synthetic nano-CaO–Al2O3–SiO2–H2O gel on the early-stage shrinkage performance of alkali-activated slag mortars. Materials (Basel). https://doi.org/10.3390/ma11071128
Barbosa TR, Foletto EL, Dotto GL, Jahn SL (2018) Preparation of mesoporous geopolymer using metakaolin and rice husk ash as synthesis precursors and its use as potential adsorbent to remove organic dye from aqueous solutions. Ceram Int 44:416–423. https://doi.org/10.1016/j.ceramint.2017.09.193
Luukkonen T, Heponiemi A, Runtti H, Pesonen J, Yliniemi J, Lassi U (2019) Application of alkali-activated materials for water and wastewater treatment: a review. Rev Environ Sci Biotechnol 18:271–297. https://doi.org/10.1007/s11157-019-09494-0
Franus W, Wdowin M, Franus M (2014) Synthesis and characterization of zeolites prepared from industrial fly ash. Environ Monit Assess 186:5721–5729. https://doi.org/10.1007/s10661-014-3815-5
Sing KSW, Reporting physisorption data for gas, solid systems with special reference to the determination of surface area and porosity (Recommendations, (1984) Pure Appl. Chem 57(1985):603–619
Sing KSW, Williams RT (2004) Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt Sci Technol 22:773–782. https://doi.org/10.1260/0263617053499032
Kesraoui-Ouki S, Cheeseman CR, Perry R (1994) Natural zeolite utilisation in pollution control: A review of applications to metals’ effluents. J Chem Technol Biotechnol 59:121–126. https://doi.org/10.1002/jctb.280590202
Othman MR, Rasid NM, Fernando WJN (2006) Mg-Al hydrotalcite coating on zeolites for improved carbon dioxide adsorption. Chem Eng Sci 61:1555–1560. https://doi.org/10.1016/j.ces.2005.09.011
Acharya H, Srivastava SK, Bhowmick AK (2007) Synthesis of partially exfoliated EPDM/LDH nanocomposites by solution intercalation: structural characterization and properties. Compos Sci Technol 67:2807–2816. https://doi.org/10.1016/j.compscitech.2007.01.030
Salih MA, Abang Ali AA, Farzadnia N (2014) Characterization of mechanical and microstructural properties of palm oil fuel ash geopolymer cement paste. Constr Build Mater 65:592–603. https://doi.org/10.1016/j.conbuildmat.2014.05.031
Alrefaei Y, Wang YS, Dai JG (2019) The effectiveness of different superplasticizers in ambient cured one-part alkali activated pastes. Cem Concr Compos 97:166–174. https://doi.org/10.1016/j.cemconcomp.2018.12.027
Puertas F, Torres-Carrasco M (2014) Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cem Concr Res 57:95–104. https://doi.org/10.1016/j.cemconres.2013.12.005
Hartmann A, Khakhutov M, Buhl JC (2014) Hydrothermal synthesis of CSH-phases (tobermorite) under influence of Ca-formate. Mater Res Bull 51:389–396. https://doi.org/10.1016/j.materresbull.2013.12.030
Mainganye D, Ojumu TV, Petrik L (2013) Synthesis of zeolites Na-P1 from South African coal fly ash: Effect of impeller design and agitation. Materials (Basel) 6:2704–2089. https://doi.org/10.3390/ma6052074
Fernández-Jiménez A, Palomo A (2005) Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater 86:207–214. https://doi.org/10.1016/j.micromeso.2005.05.057
Khalid HR (2018) Synthesis and characterization of mesoporous geopolymer solid adsorbents containing nano-crystalline zeolites. Korea Advanced Institute of Science and Technology (KAIST)
Heriyanto, Heraldy E, Nugrahaningtyas KD (2018) X-ray diffraction and fourier transform infrared study of Ca–Mg–Al hydrotalcite from artificial brine water with synthesis hydrothermal treatments. IN: IOP Conf. Ser. Mater. Sci. Eng. 333. doi:https://doi.org/10.1088/1757-899X/333/1/012006
PSM (2018) Nanostructural evolution of binder gel in alkali-activated cementitious materials exposed to extreme environments
Sitarz M, Mozgawa W, Handke M (1999) Rings in the structure of silicate glasses. J Mol Struct 511–512:281–285. https://doi.org/10.1016/S0022-2860(99)00169-6
Criado M, Fernández-Jiménez A, Palomo A (2007) Alkali activation of fly ash: Effect of the SiO2/Na2O ratio. Part I: FTIR study, Microporous Mesoporous Mater 106:180–191. https://doi.org/10.1016/j.micromeso.2007.02.055
Lee NK, Koh KT, Kim MO, An GH, Ryu GS (2017) Physicochemical changes caused by reactive MgO in alkali-activated fly ash/slag blends under accelerated carbonation. Ceram Int 43:12490–12496. https://doi.org/10.1016/j.ceramint.2017.06.119
Acknowledgements
This study was supported by a Grant from the National Research Foundation of Korea (NRF) (2018R1A2A1A05076894) funded by the Korea government (MSIT), and a Grant (19CTAP-C143331-02) from Technology Advancement Research Program (TARP) funded by Ministry of Land, Infrastructure and Transport of Korean government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Khalid, H.R., Park, S.M. et al. MgO-induced phase variation in alkali-activated binders synthesized under hydrothermal conditions. Mater Struct 54, 111 (2021). https://doi.org/10.1617/s11527-021-01689-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-021-01689-8