Abstract
Local environments have strict influence over (bio)mineralization in calcifying systems. This snapshot review discusses recent insights into the roles of Ca2+-macromolecule interactions on the nucleation of calcium carbonate and calcium phosphate minerals. Experimental findings combined with simulations/modeling are providing breakthrough information and raising important questions for future studies. The emerging picture is that both nucleation and growth are driven by local ordering of ions and water about the macromolecule interface, rather than broader properties or molecular class. Tuning macromolecular properties at the atomic scale thus provides opportunities for highly specific controls on mineralization; however, many limitations and challenges remain. We highlight studies employing in-situ atomic force microscopy (AFM) and transmission electron microscopy (TEM) to observe crystallization processes on or near macromolecular substrates. As the distribution and ability of these techniques increases, fundamental studies integrating experimental and computational methods will be crucial to inform a broad range of applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The persistence of crystallization processes and products through deep time and across diverse environments speaks to the significance of mineralization in the natural world. For example, organisms can create ornate, hierarchically organized biocomposites for many functions, with the most commonly recognized being skeletal features composed of calcium carbonate (CaCO3) or calcium phosphate materials (Fig. 1) [1,2,3]. However, the details of these processes are not yet well-understood or replicated by modern science. Many prominent fields employ crystalline materials and/or processes and would benefit from advanced control over mineralization.
For instance, calcium minerals are the most common source of scaling and buildup in industrial and water treatment plants [4, 5]. Calcium mineral-organic composites function as water purifiers, adsorbing heavy metals and synthetic dyes [6]. Other environmental applications include carbon capture [7, 8], soil remediation, and erosion prevention [9]. The biocompatible nature, low cost, and high catalytic efficiency of calcium minerals lend them to biomedical applications such as detection agents, bone scaffolds, and drug delivery systems [10,11,12]. In electronics, calcium minerals may aid the synthesis of more bioinert lithium-ion batteries [13]. Thus, organisms and industry alike prominently utilize these versatile materials.
Macromolecules in the organic matrix where crystallization takes place continue to be implicated as imperative to biomineralization. A general correlation between macromolecular charge (imparted by ionic functional groups) and the energy barrier to (classical) CaCO3 nucleation has been established [14]. While this macroscopic factor helps to identify roles of macromolecules in crystallization settings (e.g., providing a substrate, concentrating precursor ions, directing the location and orientation of crystals [15,16,17,18]), a mechanistic understanding on the atomic level remains vague. Such an understanding will be valuable to further comprehend biological crystallization processes and to make informed, directed decisions when designing new materials.
Generally, by classical nucleation theory, nucleation occurs through the monomer-by-monomer attachment of atoms, molecules, or ions to form critical nuclei, whereas non-classical nucleation theory encompasses the proceeding of nucleation through metastable particles or precursor phases. The energetic cost of creating a new interface (the interfacial energy) is a significant consideration for both. (For more detailed descriptions, see De Yoreo et al. [19], Jin et al. [20], Putnis et al. [21], Fu wt al [22]). However, studies are increasingly suggesting an underlying systematics whereby the formation of biominerals is highly regulated by ion and water structuring at the macromolecule interface for both classical and non-classical pathways [23, 24]. This structuring can thus be influenced by macromolecular composition, conformation, and other properties. These macromolecular properties can themselves be dictated by water, ion-binding, and hydrogen-bond networks (e.g., morphology [25]), further emphasizing the significance of understanding behavior at solvated interfaces. Ca2+-macromolecule interactions are especially significant given they encompass a diverse array of biochemical processes outside of biomineralization, including cell signaling and transport [26].
The idea that hydration and ion configuration play major roles in both crystal nucleation (e.g., energetic pathways) and growth is not new; however, previous researchers lacked the high-resolution in-situ imaging techniques and computational abilities that are driving advancement within this area today. Nonetheless, studying these systems still presents complex challenges. This snapshot review aims to highlight recent studies employing liquid-phase in-situ atomic force microscopy (AFM) or transmission electron microscopy (TEM) to investigate macromolecular effects on calcium carbonate and calcium phosphate mineral systems. We use in situ broadly to describe experiments observing real-time sample dynamics—with or without external stimuli. Coupled with modeling, these powerful instruments are providing sub-nanometer scale insights into Ca2+ interactions at macromolecular interfaces. Looking toward the goal of controlled crystallization, integrated studies will be required for foundational progress.
Ion-binding at the macromolecule-solution interface
Many macromolecules (e.g., proteins and polysaccharides) at sites of mineralization contain carboxyl, sulfate, and/or phosphate functional groups [27]. The anionic nature of these molecules at physiological pH has naturally led to the idea that they concentrate cations through coulombic interactions. While electrostatics play a role in drawing cations near the macromolecule, the intensity and longevity of the interaction are typically stabilized by entropic effects [28,29,30]. CaCO3 (and other Ca mineral) ion pair formation is also water/entropy motivated [31,32,33]. Therefore, ion and macromolecule hydration must both be accounted for when considering binding mechanisms and polymer design.
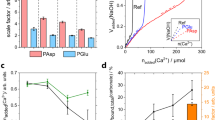
High Ca2+ affinity impacts nucleation in a variety of ways. However, a distinction must first be made between macromolecule effects in aqueous suspensions versus as a template. In solution, macromolecules are most often cited as inhibitors. Flexibility of the backbone allows macromolecules to entangle or entrap Ca2+, thus preventing it from interacting with counterions to form critical nuclei. This has been observed for both charged [34,35,36,37] and uncharged polymers [38], and in part is responsible for the success of anionic polymers as scale inhibitors [39,40,41,42]. A good example is seen in a study of calcium phosphate crystallization onto a synthetic bone scaffold by Gleeson et al. [43]. Poly(caprolactone)-b-poly(acrylic acid) (carboxylated) block copolymer (PCL-b-PAA) functionalized nanofibers promoted nucleation of a ‘mat’ of calcium phosphate (Fig. 2). When dissolved PAA was introduced to the system, crystallization was inhibited by two mechanisms (Fig. 2): (1) PAA stabilized precursor ions in solution preventing nucleation; (2) PAA created a physical barrier to the nanofiber surface [43].
A common report is that macromolecules in solution bind to crystals and impart effects on growth processes. Here, hydration and ion-binding are also significant. For example, glycosaminoglycans (GAGs) bind to calcium phosphate through a water layer rather than directly [44]. Other biopolymers bind to crystal anions to avoid the unfavorable dehydration of calcium [45, 46]. Once bound, macromolecules can block a crystal plane from solution, therefore inhibiting further growth of that facet, or promote growth by increasing rates of ion attachment to specific planes [47,48,49]. In other cases, macromolecules become incorporated into the crystal lattice, conferring changes to mechanical properties [50,51,52]. All allow for facilitation of crystal morphology and/or polymorph.
Here, we will focus on macromolecules in the form of a template or immobilized substrate. Perhaps expectedly, many charged macromolecules in the organic matrix contain unique binding domains to neutral, primarily insoluble polymers (e.g., collagen or chitin [53,54,55]). These domains may be present to allow macromolecules to be ‘tethered’ to a surface for desired crystallization effects.
Macroscopic controls of Ca2+ binding on location and timing of crystallization
The activity of immobilized PCL-b-PAA (Fig. 2) also illustrates presumably the simplest level of control macromolecules can exert over nucleation: spatial. Of course, when a surface or template is introduced to a solution, nucleation onto the template most often will be more energetically favorable due to the high energy barrier to nucleation in solution (> 100 mJ m−2) [27, 56]. However, in systems with more than one type of surface or template, a base level of control on nucleation through Ca2+ interactions is established by the type and density of macromolecular functional groups. Solution chemistry (i.e., supersaturation) can then be tuned such that crystallization occurs either in areas where certain macromolecules are present or where they are absent [14, 57]. The former is most often employed.
For example, Smeets et al. observed (by in-situ liquid-phase TEM) that polystyrene sulfonate (PSS) forms highly hydrated globules with Ca2+ [58]. These globules were immobilized on the silicon nitride TEM window. Nucleation of amorphous calcium carbonate (ACC) was then controlled by diffusion of CO32− into the TEM cell and Ca-PSS globules resulting in ACC particles solely present in/on the globules. Vaterite only began to nucleate on the Si3N4 surface when the supersaturation in free solution was high enough due to the continued supply of CO32−.
In the above case, Ca-PSS globules were formed before any CO32− was added to the system. Therefore, the onset of nucleation was not dependent on the time it took for the globules to form. In systems where both Ca2+ and anion are present, macromolecule affinity to Ca2+ permits control over nucleation timing. In environments below or with low supersaturations, strong Ca2+ binders are often considered inhibitors due to delaying induction time (i.e., max Ca2+ binding must be reached before nucleation begins) [59, 60]. Once a region of high saturation is present, the same macromolecules promote nucleation. This has been observed for different carrageenans. l-carrageenan (1.5 SO3− per monosaccharide) delayed CaCO3 nucleation the longest—over i-carrageenan (1 SO3− per monosaccharide) and k-carrageenan (0.5 SO3− per monosaccharide—but exhibited the highest crystal density [59]. These seemingly contradicting phenomena may explain disparities in the literature where the same molecule is designated as an inhibitor by some but a promoter by others.
In calcifying marine organisms, the concentration of Ca2+ ions by macromolecules can drive control over internal saturation state to direct CaCO3 placement and timing. For instance, the thickness and composition of the external polysaccharide coating of coccolithophores (marine algae) are linked to their internal chemical environment (supersaturation and potentially carbon speciation) as well as historic oceanic CO2 levels [61,62,63,64,65]. In-situ cryoTEM studies have recently shown how coccolith base plates associate with natural and synthetic carboxylated macromolecules to assemble Ca2+ at the coccolith base plate surface which decreases Ca2+ concentration in solution (Fig. 3) [66]. Vesicles within the coccolithophore—often also composed of or containing charged polysaccharides—then transport ions to mineralization locales [67]. These studies were among the first to reveal that physical confinement of crystallization settings can be equally important as solution conditions in nano-environments, exemplifying the power of in-situ TEM methods and the significance of control over Ca2+ interactions. The results also provide insight into the inconsistent findings of benchtop studies investigating the effect of pH or CO2 concentration on coccolith size and morphology [68,69,70,71,72]. Further studies are working to resolve how polysaccharide-baseplate-internal chemical environment relations regulate the unique, complex morphologies exhibited by coccoliths [73].

Reprinted from Krounbi et al., Chem. Mater. (2021) with permission. Copyright 2021 American Chemical Society
CryoTEM images (a, c, e, g, i; scale bar 1 μm) and 3D volume rendering of data (b, d, f, h, J; scale bar 0.5 μm) of baseplates (purple) and associated macromolecule-Ca2+ assemblies (synthetic: blue; natural: red).
Modulating atomic-level macromolecule-Ca2+ interactions to direct crystallization
More precise control of mineralization is enabled by the design of macromolecule configuration, conformation, and sequence. Experimental results are often contrary to general trends predicted by the hydrophilicity/phobicity or charge density of a molecule due to the organization or ‘fit’ of a molecule to the local water/ion structure [14, 28]. This is consistent with long-made assumptions that functional group sequence and/or spacing are highly significant to mineralization processes [74,75,76,77].
Akkineni et al. found that amyloid-like amelogenin nanoribbons (phosphorylated) could enhance both kinetic and thermodynamic contributions to amorphous calcium phosphate (ACP) nucleation [78]. These nanoribbons are thought to act as a scaffold for tooth enamel in natural systems. Using in-situ AFM, they measured the rate of ACP onto the ribbons and found phosphoryl groups on the nanoribbons increased nucleation rate. The authors postulated this was due to increased Ca2+ binding duration to the surface and/or higher ion exchange rates due to the charged groups. By applying classical nucleation theory to measured rates, they further determined the nanoribbons presented surprisingly low interfacial energy (1.4–20 mJ m−2) compared to collagen (40 mJ m−2) and therefore a low free energy barrier to nucleation. Traditionally, anionic functionalities are thought to increase interfacial energy (relative to neutral macromolecules) due to their hydrophilicity [14, 27]. However, few nucleation studies have been conducted involving phosphorylated macromolecules despite their biological significance [79, 80]. To resolve these findings, Akkineni et al. applied molecular dynamics (MD). Evidence from AFM and X-ray diffraction measurements indicated the nanoribbons self-assembled into a b-sheet. The simulations established this was the most stable conformation and showed the spacing of phosphoryl groups in the b-sheet matched the periodicity of ACP [78]. The interfacial energy was thus lower due to providing a favorable crystal nucleus (or crystal nucleus building blocks) binding (Fig. 4a) [78, 81].
The above results indicate energetics of crystallization can be tuned to control nucleation rate and polymorph through matching macromolecule conformation to desired crystal lattice structure, resulting in lower interfacial energy (Fig. 4a). A breakthrough study by Davila-Hernandez et al. confirmed this for CaCO3 using designed helical repeat proteins [82]. The proteins were designed via computation to be flat surfaces containing carboxylate groups at 1-nm intervals (to match the calcite lattice). Of the many possible designs, they chose a selection expected to be best synthesized by E. coli. Using the proteins that expressed in high yield, in-situ liquid-phase TEM was performed in a supersaturated CaCO3 solution. In the absence of protein, vaterite nucleated and later transformed to calcite. Vaterite also nucleated in solutions containing non-lattice matched proteins (e.g., bovine serum albumin). The designed helical repeat proteins bypassed vaterite and nucleated predominately or exclusively calcite, due to organized Ca2+ ions at the protein interface (Fig. 4b) [82]. This group has also extensively studied the folding of macromolecules on mica surfaces [83, 84]. For example, Alberstein et al. studied the crystallization of proteins on mica and determined that a hydration layer along the surface of the mica likely played a role in templating [85]. Overall, these studies suggest macromolecule-ion binding and hydration can direct crystallization in diverse mineral settings. These studies are especially noteworthy as choosing from their library of designed proteins allowed researchers to forego a ‘trial and error’ selection of materials. Looking forward, the ability to directly model these proteins at mineral interfaces would be a pronounced achievement [86].
Macromolecules (template) that present a lattice match reduce the free energy barrier to nucleation (a); schematic of designed helical repeat proteins and how they spatially organize Ca2+, favoring specific crystal orientations (b).
Davila-Hernandez et al. further observed single protein chain interactions promoted smaller, more numerous crystals than if the protein was first incubated with Ca2+ (allowing Ca-protein assemblies to form) [82]. This is consistent with a study of ACC and phenolic polymers, where it was hypothesized that polymer substituents isolate Ca2+, allowing each isolated ion or group to start a small crystal, thus leading to a smaller crystal size but greater density [87]. The findings reveal yet another level of complexity for how macromolecules may modulate Ca2+ interactions during biomineralization.
Current limitations
The works summarized in this review demonstrate many impacts of Ca2+-macromolecule surface binding on calcium mineral nucleation. As advances and tools expand, several limitations warrant careful consideration. We briefly discuss them below.
Experimental
Liquid-phase, in-situ AFM and TEM have substantial capabilities to reveal nanoscale details of materials and processes, which is pertinent to understanding biomineralization processes [88, 89]. Despite these capabilities, both encounter analytical constraints. Sample preparation for each technique requires meticulous attention and careful substrate selection. On the practical side, technical knowledge and training are required for instrument operation, experimental design, and data interpretation. Further, few facilities have access to commercially available liquid cell in-situ holders, inhibiting in-situ liquid cell capabilities. While other thin film options, such as graphene liquid cells, are more widely available, these methods are limited by a lack of liquid flow [90]. Thus, the overall accessibility of liquid-phase in-situ AFM and TEM is low.
One major limitation of AFM is that it is a slow imaging technique [91]. AFM also presents much less lateral resolution compared to vertical [92]. While not being performed in a vacuum makes AFM well-suited for biological samples, this technique has high environmental sensitivity. Tip sensitivity and accuracy can be influenced by temperature, humidity, and vibrations [91, 92]. However, continual advancement in tip design and control mechanisms will reduce these issues. An additional disadvantage of in-situ AFM is the inability to pair it with complementary methods for elemental analysis (e.g., energy-dispersive X-ray spectroscopy [93]), which can be done using TEM.
With only remarkable spatial resolution, TEM lacks depth profiling and only provides 2D information [92]. This prevents obtaining 3D architectures or discerning distance between materials on the z-axis. A second limitation is beam effects. The high-energy electrons can induce changes in temperature, pH, and material stability potentially destroying the sample [94,95,96]. Determining if observed phenomena are driven by the electron beam or ‘true’ processes is imperative to interpreting results and bulk solution observations. This is also true for confinement effects [97], especially in thin film systems [98]. Beam effects can be moderated through electron dose, dose rate, and acceleration voltage [96, 99], and computer models/machine learning allows particular beam effects to be predicted [100]. Ultimately, they are an unavoidable aspect of this technique, but improved acquisition measures (e.g., direct electron detectors) will continue to help reduce beam effects and improve resolution (spatial and temporal).
Perhaps the greatest limitation presented by TEM to biomineralization studies is the difficulty of imagining biomacromolecules. Due to their low electron density [101,102,103], very weak contrast is seen between the surrounding background and proteins/polysaccharides, impeding direct observation of the interface. Addressing this challenge will require specialized and creative approaches to sample preparation and imaging conditions.
Overall, experimental ‘restrictions’ highlight the necessity for complementary techniques to fully elucidate the intricacies of materials at the sub-nanometer or atomic scale.
Computation
Modeling biomineral systems provides invaluable insights but poses many computational challenges due to the complex interplay of biological, chemical, and physical processes involved. Even at the simplest scale, modeling water continues to be a problematic task. At present, water is often considered a continuum, which is known to be an incorrect assumption at interfaces [104]. Moreover, mechanisms of conversion between solvent-separated, solvent-shared, and contact ion pairs (as well as water movement between solvation shells) are difficult and resource-intensive to simulate accurately [105, 106]. Increasing the level of theory applied significantly increases computational costs and restricts system size. Error estimation and limited experimental data also contribute. The issue becomes impressively complex as other ions and interfaces are introduced to the system [33, 105,106,107]. Further, scaling up from the atomic level to bulk solution expounds uncertainty and error. This is, in part, due to the parallel uncertainty when relating experimental TEM observations and bulk solution behavior. Tackling these and additional computational limitations will require efficient algorithms, parallel computing techniques, and advanced simulation methodologies.
Conclusions
Calcium-water-macromolecule interactions encompass a diverse array of biochemical processes, and there is increasing evidence that anions play a larger role than previously thought [24, 104, 108]. Despite the complexity of these systems (and studying them), recent advancements in imaging techniques and computational modeling have provided unprecedented insights into the atomic-level mechanisms governing mineralization. By integrating experimental and computational approaches, we can continue to unravel the intricacies of biomineralization, poising researchers to leverage this knowledge for the design and control of crystallization for diverse applications.
Fully resolving the dynamic nature of biomineralization—influenced by factors like genetic regulation, environmental conditions, and biochemical signaling—from atomic-level interactions to tissue-level organization will require a concerted effort from interdisciplinary researchers to develop scalable, robust, and biologically realistic approaches. Ultimately, these efforts not only advance our fundamental understanding of natural processes but are also paramount in realizing the full potential of designed mineral-organic composites for societal benefit.
References
K. Simkiss, K.M. Wilbur, Biomineralization (Elsevier, Amsterdam, 1989)
S. Mann, Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry (Oxford University Press, Oxford, 2001)
C.C. Perry, Silicification: The processes by which organisms capture and mineralize silica, in Biomineralization. ed. by P.M. Dove, J.J. DeYoreo, S. Weiner (Mineralogical Society of America, Chantilly, 2003), pp.291–327
M.S. Reddy, Front. Microbiol. (2013). https://doi.org/10.3389/fmicb.2013.00314
T. Fungene, S. Ndlovu, E. Matinde, Sep. Sci. Technol. 58, 7 (2023). https://doi.org/10.1080/01496395.2023.2189051
A. Salama, Int. J. Biol. Macromol. (2019). https://doi.org/10.1016/j.ijbiomac.2019.01.130
V. Achal, A. Mukherjee, D. Kumari, Q. Zhang, Eaarth-Sci. Rev. (2015). https://doi.org/10.1016/j.earscirev.2015.05.008
H. Zhang, T. Zhang, J.C. Zang, C.Y. Lv, G.H. Zhao, Food Hydrocoll. (2022). https://doi.org/10.1016/j.foodhyd.2022.107693
M.Z. Song, T.Y. Ju, Y. Meng, S.Y. Han, L. Lin, J.G. Jiang, Chemosphere (2022). https://doi.org/10.1016/j.chemosphere.2021.133229
J. Jeong, J.H. Kim, J.H. Shim, N.S. Hwang, C.Y. Heo, Biomater. Res. 23, 1 (2019). https://doi.org/10.1186/s40824-018-0149-3
P. Fadia, S. Tyagi, S. Bhagat, A. Nair, P. Panchal, H. Dave, S. Dang, S. Singh, 3 Biotech (2021). https://doi.org/10.1007/s13205-021-02995-2
O.A. Osuchukwu, A. Salihi, I. Abdullahi, P.O. Etinosa, D.O. Obada, Mater. Chem. Phys. (2023). https://doi.org/10.1016/j.matchemphys.2023.127434
T. Wang, N. Liu, H. Zhou, M.J. Chen, Micro Nano Lett. (2023). https://doi.org/10.1049/mna2.12168
A.J. Giuffre, L.M. Hamm, N. Han, J.J. De Yoreo, P.M. Dove, Proc. Natl. Acad. Sci. USA 110, 23 (2013). https://doi.org/10.1073/pnas.1222162110
L. Addadi, S. Weiner, Angew Chem. Int. Ed. (1992). https://doi.org/10.1002/anie.199201531
F. Marin, G. Luquet, B. Marie, D. Medakovic, Molluscan shell proteins: Primary structure, origin, and evolution, in Current Topics in Developmental Biology. ed. by G.P. Schatten (Academic Press, Cambridge, 2008)
S. Tambutte, M. Holcomb, C. Ferrier-Pages, S. Reynaud, E. Tambutte, D. Zoccola, D. Allemand, J. Exp. Mar. Biol. Ecol. 408, 1–2 (2011). https://doi.org/10.1016/j.jembe.2011.07.026
Y. Zhao, Z. Han, H. Yan, H. Zhao, M.E. Tucker, M. Han, G. Mao, J. Yin, Geomicrobiol. J. (2020). https://doi.org/10.1080/01490451.2019.1695023
J.J. De Yoreo, P. Gilbert, N. Sommerdijk, R.L. Penn, S. Whitelam, D. Joester, H.Z. Zhang, J.D. Rimer, A. Navrotsky, J.F. Banfield, A.F. Wallace, F.M. Michel, F.C. Meldrum, H. Colfen, P.M. Dove, Science. 349, 6247 (2015). https://doi.org/10.1126/science.aaa6760
B. Jin, Z. Liu, R. Tang, CrystEngComm (2020). https://doi.org/10.1039/D0CE00480D
C.V. Putnis, L. Wang, E. Ruiz-Agudo, C. Ruiz-Agudo, F. Renard, Crystallization via Nonclassical Pathways: Nanoscale Imaging of Mineral Surfaces. In Crystallization via Nonclassical Pathways Volume 2: Aggregation, Biomineralization, Imaging & Application, (American Chemical Society, 1383:1–35 2021)
H. Fu, X. Gao, X. Zhang, L. Ling, Cryst. Growth Des. (2022). https://doi.org/10.1021/acs.cgd.1c01084
N.F.A. van der Vegt, K. Haldrup, S. Roke, J.R. Zheng, M. Lund, H.J. Bakker, Chem. Rev. 116, 13 (2016). https://doi.org/10.1021/acs.chemrev.5b00742
H. Lu, Y.-C. Huang, J. Hunger, D. Gebauer, H. Coelfen, M. Bonn, J. Am. Chem. Soc. (2021). https://doi.org/10.1021/jacs.0c11976
R. Roy, N.A. Jonniya, P. Kar, J. Phys. Chem. B 126, 21 (2022). https://doi.org/10.1021/acs.jpcb.2c01807
J. Karlstad, Y. Sun, B.B. Singh, Adv. Exp. Med. Biol. (2012). https://doi.org/10.1007/978-94-007-2888-2_6
B.M. Knight, K.J. Edgar, J.J. De Yoreo, P.M. Dove, Biomacromolecules (2023). https://doi.org/10.1021/acs.biomac.2c01394
C.G. Sinn, R. Dimova, M. Antonietti, Macromolecules. 37, 9 (2004). https://doi.org/10.1021/ma030550s
W.R. Archer, C.M.B. Gallagher, V. Vaissier Welborn, M.D. Schulz, Phys. Chem. Chem. Phys. 24, 6 (2022). https://doi.org/10.1039/D1CP05263B
V.V. Welborn, W.R. Archer, M.D. Schulz, J. Chem. Inf. Model. 63, 7 (2023). https://doi.org/10.1021/acs.jcim.2c01048
M. Kellermeier, P. Raiteri, J.K. Berg, A. Kempter, J.D. Gale, D. Gebauer, ChemPhysChem 17, 21 (2016). https://doi.org/10.1002/cphc.201600653
H.J. Li, D. Yan, H.Q. Cai, H.B. Yi, X.B. Min, F.F. Xia, Phys. Chem. Chem. Phys. 19, 18 (2017). https://doi.org/10.1039/c7cp00428a
Y. Li, H. Zeng, H. Zhang, Mater. Genome Eng. Adv. 1, 1 (2023). https://doi.org/10.1002/mgea.4
A. Jada, A. Verraes, Colloids Surfaces a-Physicochem. Eng. Asp. 219, 1–3 (2003)
J.G. Yu, M. Lei, B. Cheng, X.J. Zhao, J. Solid State Chem. (2004). https://doi.org/10.1016/j.jssc.2003.08.017
S. Ouhenia, D. Chateigner, M.A. Belkhir, E. Guilmeau, C. Krauss, J. Cryst. Growth. 310, 11 (2008). https://doi.org/10.1016/j.jcrysgro.2008.02.006
A. Jada, A. Verraes, A. Aue, C. Ducroquetz, e-Polym. (2009)
M. Cole, G. Eggleston, Y.J. Wang, Food Chem. (2019). https://doi.org/10.1016/j.foodchem.2018.09.076
X.R. Guo, F.X. Qiu, K. Dong, X. Zhou, J. Qi, Y. Zhou, D.Y. Yang, J. Indust Eng. Chem. 18, 6 (2012). https://doi.org/10.1016/j.jiec.2012.06.015
R. Macedo, N.D. Marques, L.C.S. Paulucci, J.V.M. Cunha, M.A. Villetti, B.B. Castro, R.D. Balaban, Carbohydr. Polym. (2019). https://doi.org/10.1016/j.carbpol.2019.03.082
Y.A. Maher, M.E.A. Ali, H.E. Salama, M.W. Sabaa, Arab. J. Chem. 13, 1 (2020). https://doi.org/10.1016/j.arabjc.2018.08.006
G. Bolivar, M. Colina, B. Delgado, E. Mendizabal, J. Mex Chem. Soc. 65, 1 (2021). https://doi.org/10.29356/jmcs.v65i1.1429
S.E. Gleeson, S. Kim, T. Yu, M. Marcolongo, C.Y. Li, ACS Appl. Bio Mater. (2022). https://doi.org/10.1021/acsabm.2c00583
S.E.R. Hernandez, I. Streeter, N.H. de Leeuw, Phys. Chem. Chem. Phys. (2015). https://doi.org/10.1039/c5cp02630j
M.J. Yang, S.L.S. Stipp, J. Harding, Cryst. Growth Des. (2008). https://doi.org/10.1021/cg800508t
R.K. Puthanveettil, Y. Lee, J. Heo, M.J. Kim, Adv. Powder Technol. 34, 12 (2023). https://doi.org/10.1016/j.apt.2023.104249
G. Fu, S.R. Qiu, C.A. Orme, D.E. Morse, J.J. De Yoreo, Adv. Mater. (2005). https://doi.org/10.1002/adma.200500633
S. Elhadj, J.J. De Yoreo, J.R. Hoyer, P.M. Dove, Proc. Natl. Acad. Sci. USA (2006). https://doi.org/10.1073/pnas.0605748103
C.L. Chen, K.M. Bromley, J. Moradian-Oldak, J.J. DeYoreo, J. Am. Chem. Soc. 133, 43 (2011). https://doi.org/10.1021/ja206849c
S.Y. Cho, Y.S. Yun, S. Lee, D. Jang, K.Y. Park, J.K. Kim, B.H. Kim, K. Kang, D.L. Kaplan, H.J. Jin, Nat. Commun. (2015). https://doi.org/10.1038/ncomms8145
Y.Y. Kim, J.D. Carloni, B. Demarchi, D. Sparks, D.G. Reid, M.E. Kunitake, C.C. Tang, M.J. Duer, C.L. Freeman, B. Pokroy, K. Penkman, J.H. Harding, L.A. Estroff, S.P. Baker, F.C. Meldrum, Nat. Mater. 15, 8 (2016). https://doi.org/10.1038/nmat4631
C.T. Hendley, L.A. Fielding, E.R. Jones, A.J. Ryan, S.P. Armes, L.A. Estroff, J. Am. Chem. Soc. 140, 25 (2018). https://doi.org/10.1021/jacs.8b03828
H.A. Lowenstam, S. Weiner, On Biomineralization (Oxford University Press, Oxford, 1989)
P. Willmer, Invertebrate relationships. Patterns in animal evolution. (Cambridge University: 1990)p i-xiv, 1-400
H. Ehrlich, Int. Geol. Rev. 52, 7–8 (2010).
W.J.E.M. Habraken, J. Tao, L.J. Brylka, H. Friedrich, L. Bertinetti, A.S. Schenk, A. Verch, V. Dmitrovic, P.H.H. Bomans, P.M. Frederik, J. Laven, P. van der Schoot, B. Aichmayer, G. de With, J.J. DeYoreo, Nat. Commun. (2013).
A.R. Nielsen, S. Jelavić, D. Murray, B. Rad, M.P. Andersson, M. Ceccato, A.C. Mitchell, S.L.S. Stipp, R.N. Zuckermann, K.K. Sand, Cryst. Growth Des. 20, 6 (2020). https://doi.org/10.1021/acs.cgd.0c00029
P.J.M. Smeets, K.R. Cho, R.G.E. Kempen, N.A.J.M. Sommerdijk, J.J. De Yoreo, Nat. Mater. (2015). https://doi.org/10.1038/nmat4193
R. Fried, Y. Mastai, J. Cryst. Growth. 338, 1 (2012). https://doi.org/10.1016/j.jcrysgro.2011.09.044
S.Y. Bahn, B.H. Jo, B.H. Hwang, Y.S. Choi, H.J. Cha, Cryst. Growth Des. 15, 8 (2015). https://doi.org/10.1021/acs.cgd.5b00275
K.E. Chave, Science. 148, 3678 (1965). https://doi.org/10.1126/science.148.3678.1723
P.J. Troy, Y.H. Li, F.T. Mackenzie, Aquat. Geochem. 3, 1 (1997). https://doi.org/10.1023/a:1009652821575
S.D. Rokitta, B. Rost, Limnol. Oceanogr. 57, 2 (2012). https://doi.org/10.4319/lo.2012.57.2.0607
C. Brownlee, G.L. Wheeler, A.R. Taylor, Sem Cell. Dev. Biol. (2015). https://doi.org/10.1016/j.semcdb.2015.10.027
E.B. Wilkes, R.B.Y. Lee, H.L.O. McClelland, R.E.M. Rickaby, A. Pearson, Org. Geochem. (2018). https://doi.org/10.1016/j.orggeochem.2018.02.006
L. Krounbi, K. Hedderick, Z. Eyal, L. Aram, E. Shimoni, L.A. Estroff, A. Gal, Chem. Mater. (2021). https://doi.org/10.1021/acs.chemmater.0c04668
Y. Kadan, F. Tollervey, N. Varsano, J. Mahamid, A. Gal, Gal, Proc. Natl. Acad. Sci. USA 118, 46 (2021). https://doi.org/10.1073/pnas.2025670118
Y. Shiraiwa, Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 136, 4 (2003). https://doi.org/10.1016/s1096-4959(03)00221-5
L.T. Bach, C. Bauke, K.J.S. Meier, U. Riebesell, K.G. Schulz, Biogeosciences. 9, 8 (2012). https://doi.org/10.5194/bg-9-3449-2012
A. Engel, C.C. Novoa, M. Wurst, S. Endres, T.T. Tang, M. Schartau, C. Lee, Mar. Ecol. Prog Ser. (2014). https://doi.org/10.3354/meps10824
R.E. Diner, I. Benner, U. Passow, T. Komada, E.J. Carpenter, J.H. Stillman, Mar. Biol. 162, 6 (2015). https://doi.org/10.1007/s00227-015-2669-x
R.E.M. Rickaby, M. Hermoso, R.B.Y. Lee, B.D. Rae, A.M.C. Heureux, C. Balestreri, L. Chakravarti, D.C. Schroeder, C. Brownlee, Deep-Sea Res. Part Ii-Topical Stud Oceanograp. (2016). https://doi.org/10.1016/j.dsr2.2016.02.010
Z. Eyal, L. Krounbi, O. Ben Joseph, E.M. Avrahami, I. Pinkas, H. Peled-Zehavi, A. Gal, Acta Biomater. (2022). https://doi.org/10.1016/j.actbio.2022.06.027
J.I. Arias, C. Jure, J.P. Wiff, M.S. Fernandez, V. Fuenzalida, Arias in Effect of Sulfate Content of Biomacromolecules on the Crystallization of Calcium Carbonate, Symposia on Physical Characterization of Biological Materials and Systems/Polymeric Biomaterials for Tissue Engineering/BioInspired (Materials-Moving Toward Complexity, Boston, 2001), pp.26–29
J.L. Arias, M.S. Fernandez, Mater. Charact. 50, 2–3 (2003). https://doi.org/10.1016/s1044-5803(03)00088-3
J.L. Arias, A. Neira-Carrillo, J.I. Arias, C. Escobar, M. Bodero, M. David, M.S. Fernandez, J. Mater. Chem. 14, 14 (2004). https://doi.org/10.1039/b401396d
A. Neira-Carrillo, S. Pillai, R.K. Pai, J. Chil. Chem. Soc. 59, 1 (2014). https://doi.org/10.4067/s0717-97072014000100014
S. Akkineni, C. Zhu, J.J. Chen, M. Song, S.E. Hoff, J. Bonde, J.H. Tao, H. Heinz, S. Habelitz, J.J. De Yoreo, Proc. Natl. Acad. Sci. USA (2022). https://doi.org/10.1073/pnas.2106965119
Y. Wu, J.L. Ackerman, E.S. Strawich, C. Rey, H.M. Kim, M.J. Glimcher, Calcif Tissue Int. (2003). https://doi.org/10.1007/s00223-002-1068-8
C. Paulo, J.P.L. Kenney, P. Persson, M. Dittrich, Geosciences. 8, 12 (2018)
L.M. Hamm, A.J. Giuffre, N. Han, J. Tao, D. Wang, J.J. De Yoreo, P.M. Dove, Proc. Natl. Acad. Sci. USA 111, 4 (2014). https://doi.org/10.1073/pnas.1312369111
F.A. Davila-Hernandez, B. Jin, H. Pyles, S. Zhang, Z. Wang, T.F. Huddy, A.K. Bera, A. Kang, C.-L. Chen, J.J. De Yoreo, D. Baker, Nat. Commun. 14, 1 (2023). https://doi.org/10.1038/s41467-023-43608-1
X. Ma, S. Zhang, F. Jiao, C.J. Newcomb, Y. Zhang, A. Prakash, Z. Liao, M.D. Baer, C.J. Mundy, J. Pfaendtner, A. Noy, C.-L. Chen, J.J. De Yoreo, Nat. Mater. 16, 7 (2017). https://doi.org/10.1038/nmat4891
H. Pyles, S. Zhang, J.J. De Yoreo, D. Baker, Nature. 571, 7764 (2019). https://doi.org/10.1038/s41586-019-1361-6
R.G. Alberstein, J.L. Prelesnik, E. Nakouzi, S. Zhang, J.J. De Yoreo, J. Pfaendtner, F.A. Tezcan, C.J. Mundy, J. Phys. Chem. Lett. 14, 1 (2023). https://doi.org/10.1021/acs.jpclett.2c02948
H. Pyles, F.A. Davila-Hernandez, B. Jin, A. Saragovi, S. Zhang, S. Schmid, L. Liu, J. Du, J.J. De Yoreo, D. Baker, Designing Interfaces Between Inorganic Crystals and De Novo Proteins. In 2023 Materials Research Society Fall Meeting and Exhibit, Boston (2023)
Y. Nakanishi, B.H. Cheng, J.J. Richardson, H. Ejima, RSC Adv. 13, 43 (2023). https://doi.org/10.1039/d3ra04791a
E. Beniash, A. Dey, N. Sommerdijk, Transmission electron microscopy in biomineralization research: advances and challenges, in Biomineralization Sourcebook, 1st edn., ed. by E. DiMasi, L.B. Gower (CRC, Boca Raton, 2014)
C. Zeng, C. Vitale-Sullivan, X. Ma, Minerals. 7, 9 (2017)
S. Pu, C. Gong, A.W. Robertson, Royal Soc. Open. Sci. 7, 1 (2020). https://doi.org/10.1098/rsos.191204
L. Zhou, M. Cai, T. Tong, H. Wang, Sensors. 17, 4 (2017)
P. Eaton, P. Quaresma, C. Soares, C. Neves, M.P. de Almeida, E. Pereira, P. West, Ultramicroscopy (2017). https://doi.org/10.1016/j.ultramic.2017.07.001
D.J. Kelly, M. Zhou, N. Clark, M.J. Hamer, E.A. Lewis, A.M. Rakowski, S.J. Haigh, R.V. Gorbachev, Nano Lett. (2018). https://doi.org/10.1021/acs.nanolett.7b04713
N. Jiang, Rep. Prog Phys. 79, 1 (2016). https://doi.org/10.1088/0034-4885/79/1/016501
T. Susi, J.C. Meyer, J. Kotakoski, Nat. Rev. Phys. (2019). https://doi.org/10.1038/s42254-019-0058-y
W.J. Zheng, D. Lee, H.M. Zheng, MRS Bull. (2024). https://doi.org/10.1557/s43577-024-00661-5
B. Jin, Z. Liu, C. Shao, J. Chen, L. Liu, R. Tang, J.J. De Yoreo, Cryst. Growth Des. (2021). https://doi.org/10.1021/acs.cgd.1c00503
K. He, M. Sawczyk, C. Liu, Y. Yuan, B. Song, R. Deivanayagam, A. Nie, X. Hu, V.P. Dravid, J. Lu, C. Sukotjo, Y.- Lu, P. Král, T. Shokuhfar, R. Shahbazian-Yassar, Sci. Adv. (2020). https://doi.org/10.1126/sciadv.aaz7524
A. Velazco, A. Béché, D. Jannis, J. Verbeeck, Ultramicroscopy (2022). https://doi.org/10.1016/j.ultramic.2021.113398
C.N. Valencia, W.B. Lomholdt, M.H.L. Larsen, T.W. Hansen, J. Schiotz, Nanoscale (2024). https://doi.org/10.1039/d3nr05220f
M.V. Shapovalov, R.L. Dunbrack Jr., Proteins: Struct. Funct. Bioinf. 66, 2 (2007)
M. Kurudirek, T. Onaran, Radiat. Phys. Chem. (2015). https://doi.org/10.1016/j.radphyschem.2015.03.034
B. Cuevas-Zuviría, L.F. Pacios, J. Chem. Inf. Model. 60, 8 (2020). https://doi.org/10.1021/acs.jcim.0c00197
V. Marinova, S. Yeandel, V. Fantauzzo, C. Freeman, J. Harding, Nucleation and Growth of Minerals on Self-Assembled Monolayers. In 2023 Materials Research Society Fall Meeting and Exhibit, Boston, (2023)
J.-N. Boyn, E.A. Carter, J. Phys. Chem. B 127, 50 (2023). https://doi.org/10.1021/acs.jpcb.3c05369
J.-N. Boyn, E.A. Carter, J. Am. Chem. Soc. 145, 37 (2023). https://doi.org/10.1021/jacs.3c06182
D. Laage, T. Elsaesser, J.T. Hynes, Chem. Rev. 117, 16 (2017). https://doi.org/10.1021/acs.chemrev.6b00765
S. Yeandel, V. Marinova, E. Armstrong, C. Freeman, J. Harding, The Impact of Local Structure and Dynamics on the Free Energy of Aqueous Mineral Interfaces. In 2023 Materials Research Society Fall Meeting and Exhibit, Boston, (2023)
Funding
The authors received funding support from the US DOE Office of Basic Energy Sciences (OBES), Division of Chemical Sciences, Geosciences and Biosciences through award DE FG02-00ER15112 during the preparation of this manuscript. We thank Jingshan Du for the invitation to write this snapshot review.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception, design, and literature search. The first draft of the manuscript was written by B.M.K., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knight, B.M., McCutchin, C.A. Modulating ion-binding at macromolecular interfaces during (bio)mineralization: A snapshot review for calcium carbonate and calcium phosphate systems. MRS Advances (2024). https://doi.org/10.1557/s43580-024-00860-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43580-024-00860-x