Abstract
The effect of temperature (T) is studied on the swelling of model poly(vinyl acetate) (PVAc) gels in isopropyl alcohol. The theta temperature Θ of these gels, at which the second osmotic virial coefficient A2 vanishes, is close to that of the corresponding high molecular mass polymer solution without cross-links so that solvent quality may be defined in the same way as the corresponding precursor polymer solution. We quantified the swelling and deswelling of PVAc gels relative to their size at the theta temperature, and also determined the effect of T on the shear modulus of these gels. It was found that both swelling and deswelling data could be reduced to a universal scaling equation of the same general form as derived from renormalization group (RG) theory for flexible linear polymer chains in solutions.
Graphical abstract
Variaton of the volumetric swelling factor as a function of the reduced temperature for PVAc gels swollen in isopropyl alcohol.

Similar content being viewed by others
Introduction
Gels have recently been of particular interest in connection with their use in various materials science applications. New types of synthetic gels have been developed where the chemistry has been tailored to meet particular end uses [1, 2].
Early works aimed at understanding the thermodynamics and mechanical behavior of rubber. Modeling this type of material where the “dry rubber” was naturally chosen as the reference state gave rise to the classical theories of rubber elasticity developed by James and Guth [3,4,5,6], later developed further by many other researchers to describe important practical problems of network swelling [7]. These well-known theories describe the swelling of cross-linked polymers by combining the classical rubber elasticity model with the mean field Flory–Huggins theory of the free energy of mixing polymers with solvents [8].
It is generally appreciated that the scaling theory of polymer solutions developed by de Gennes [9], and its numerous later extensions, represented an important advance over the early mean field theories of polymer swelling. The thermodynamic properties deduced by the scaling theory addressing fluctuation effects are not generally consistent with the Flory–Huggins based theory of polymer excluded volume interactions [10, 11]. The original scaling theory of polymer solutions was mainly confined to the good solvent limit where these fluctuation effects are largest, although scaling arguments have often been applied to the theta solvent condition in which the chain dimensions are approximately “ideal”, i.e., flexible polymer chains can be described reasonably by gaussain chains. However, the treatment of the swelling and elasticity of chemically cross-linked polymer networks made of flexible chains in solution as a function of solvent quality requires a general framework that must go beyond both the Flory–Huggins and scaling theories [12].
We focus on the effect of variable excluded volume interaction strength or “solvent quality” on network swelling and shear moduli based on experiments performed on well-characterized model polymer gels in which the cross-link density and T are varied over a wide range. We utilize these observations, acquired over a period of decades, to test the validity of theoretical frameworks proposed to describe the thermodynamic and elastic properties of these gels. In particular, we determine the changes in the network chain dimensions and shear modulus when the solvent quality is varied from “very good”, corresponding to T far above the theta point, to “very poor” where the solvent is ejected from the gels. We refer here to solutions that phase separate upon cooling. Many hydrogels “collapse” upon heating so that the transition to a poor solvent regime occurs upon heating rather than cooling [13, 14].
This change of swelling behavior can easily be incorporated in the swelling description described below through a consideration of the absolute value of the difference between T and θ in the definition of solvent quality). Experimental studies of swelling and chain conformation of gels made from a flexible linear polymer, poly(vinyl acetate) (PVAc), are reported and then reanalyzed within the theoretical framework described below [15].
Background
Significant progress beyond scaling theory was made on the basis of the renormalization group (RG) theory to achieve a quantitative description of the swelling and osmotic properties of flexible linear polymers under variable solvent quality conditions. Our objective is to apply results drawn from this theoretical framework to describe excluded volume effects in polymer networks cross-linked in solution. This approach allows us to avoid the assumptions of the mean field Flory–Huggins theory, which is known to provide an inadequate description of semi-dilute and dilute polymer solutions.
While there have been RG calculations for randomly cross-linked networks, these predictions have not yet been compared to experiments as in the case of linear polymers. Chremos et al. [16] studied model branched polymer networks with variable excluded volume interaction strength and compared his simulations to the RG theory with promising results. This simulation showed that the behavior of randomly branched polymers exhibited a qualitatively similar swelling behavior of polymer networks with variable solvent quality as found for linear flexible polymer chains in solution, although with somewhat different scaling exponents. This suggested to us that swelling measurements on PVAc gels might be described by the same RG framework and the present work investigates this possibility.
The PVAc networks were synthesized by cross-linking of long polymer chains in solution (see Materials and Methods) under conditions in which linear precursor chains are swollen, i.e., scaling theory describes the osmotic and elastic behavior of these materials. This corresponds to a swelling exponent ν ≈ 3/5, and the corresponding scaling exponent for the concentration dependence of the shear modulus is close to 2.25. These exponents require some explanation.
It is important to note that the swelling exponent for an equilibrium ensemble of randomly branched polymers forming a swollen network is exactly ν = ½ in three spatial dimensions [17], which implies that the osmotic and shear moduli scale with the polymer volume fraction as φ3. This scaling is clearly observed in the simulations of Chremos et al. for the size randomly branched networks and in the corresponding consistent scaling of the osmotic modulus and with the concentration correlation length scaling as, ξ ~ φ −1 [18]. This change of scaling exponents is expected from the change of the linear polymer to a branched polymer universality class. However, it is plausible to assume that cross-linking of high molecular mass polymers locks the network into a non-equilibrium state of the material before the cross-links were introduced, i.e., we hypothesize that topological constraints do not allow the network to relax to an equilibrium randomly branched polymer studied in previous simulations, but rather the networks cross-linked in semi-dilute solution should preserve the scaling properties of linear semi-dilute polymer solutions for which ν ≈ 3/5. Our observations discussed below are consistent with this hypothesis, but this “memory effect” reflecting the thermodynamic state of cross-linking clearly deserves further study before it can be uncritically accepted.
Materials and methods
Preparation of polyvinyl alcohol gels
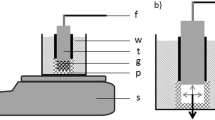
Polyvinyl alcohol (PVA) gels were made by cross-linking aqueous PVA solutions of different concentrations, c = 3.0, 6.0, 9.0, and 12.0 mass % with glutaraldehyde (GDA, Merck) at pH 1.5 at 25 °C [15]. The average chain length between neighboring cross-links was varied from 50 to 400 monomer units. Cylindrical gel samples (1 cm in diameter and 1 cm in height) were made in a mold. After gelation the gel samples were equilibrated with distilled water to remove HCl and unreacted polymeric materials.(Scheme 1).
Preparation of poly(vinyl acetate) gels
PVAc gels were prepared from PVA gels by acetylation in a mixture of acetic anhydride (40 vol %)-acetic acid (10 vol %)-pyridine (50 vol %) at 90 °C for 8 h. The details of the chemical procedure have been discussed in previous publications [15, 19, 20]. The gel samples were washed with toluene. The extent of acetylation was measured and an agreement within 1 to 2% was found between the calculated and experimentally determined values. The dry PVAc networks were swollen in isopropyl alcohol.
Shear modulus measurements
The shear modulus G of the gels was determined using a TA.XT2I HR Texture Analyser (Stable Micro Systems, UK), by uniaxial compression measurements [21]. G was estimated using the Mooney–Rivlin relation [22, 23],
where σ is the reduced stress (force per unit undeformed cross-section), λ (= L/Lo, L and Lo are the lengths of the deformed and undeformed gel specimen, respectively) is the deformation ratio and C1 and C2 are constants. In Eq. 1 the value of 2 C1 is equal to the shear modulus G of the gel. It was found that the value of C2 was approximately zero in all PVAc/isopropyl alcohol gels. This result is consistent with many previous findings reported for other swollen polymer networks.
Uniaxial compression measurements were performed at 30, 37, 45, 50, 52, 55, 60, and 70 °C on PVAc gels swollen in isopropyl alcohol. The reproducibility of the whole procedure, including gel preparation, and modulus measurements was found to be 4 to 5%.
Results and discussion
As noted above, PVAc gels have been studied as a model material in a large body of high-quality measurements. Of particular interest for the present work is a series of measurements on the mechanical and swelling properties of these networks by Horkay and Zrinyi [15, 19]. We use these data obtained over an exceptionally wide range of temperatures to investigate the effect of solvent quality on the swelling and elastic properties to derive a universal equation of state.
Influence of solvent quality on swelling of PVAc networks
Figure 1a shows the equilibrium swelling degree 1/ φe of PVAc gels as a function of T. The initial polymer concentration ranges from 3 to 12% polymer mass percent and the T varies from 30 to 70 °C. We quantify the cross-linking density by the average number of monomer units between cross-links, estimated from the stoichiometry of the cross-linking process. In this figure, the gels are designated by the mass percent of the polymer at cross-linking and the average number of monomer units between neighboring cross-links.
a Equilibrium swelling degree 1/φe for PVAc gels swollen in isopropyl alcohol as a function of T. b Volumetric swelling factor α3 for PVAc/isopropyl alcohol gels vs reduced temperature τ. The inset shows the variation of the second and third osmotic virial coefficients as a function of the T. Symbols are the same as in a
We introduce a reduced variable for the description of the swelling data based on previous studies of excluded volume effects on the swelling and mechanical properties of gels. It is generally accepted that the theta point at which binary excluded volume interactions of long flexible polymers vanishes is a natural reference T of polymer solutions. For PVAc gels in isopropyl alcohol the “theta temperature” Θ equals 52 °C, which corresponds to the T at which attractive binary excluded volume interactions compensate repulsive binary excluded volume interactions. We have previously determined A2 as a function of T for our PVAc gels in isopropyl alcohol [24], and we show its variation with T in the inset of Fig. 1b.
We next define the reduced temperature, τ = (T–Θ) / T, as a measure of “solvent quality”, where τ < 0 corresponds to a “poor solvent” and τ > > 0 to a “good solvent”. In Fig. 1b, we show the gel “swelling factor” α3 (= gel volume relative to its volume at Θ) as a function of the reduced temperature. The swelling curves cross at the reduced temperature, τ = 0, i.e., the theta point.
The natural excluded volume parameter for gels is the reduced variable, (τ/φθ) where 1/φθ is defined as the ratio of the volume of the gel under theta conditions to its value in its dry state Vgel, dry,
Then the excluded volume interaction zg is given by the relation,
where δ is a constant which varies with solvent and polymer type as in the case of flexible polymers in solution. [25]
In Fig. 2, is compared the α(zg) data for PVAc/isopropyl alcohol gels to the RG theoretical expression [26,27,28],
where (2ν–1)/φ equals 2/5 using the Flory approximation of ν ≈ 3/5 in d = 3 (the value appropriate to linear flexible chains, as described above) and the constant b is taken to be empirical. u* is a characteristic constant of the RG theory describing the width of linear variation of α on z that can be adsorbed into z in comparisons to experiment. More generally, these exponents are well described by Eq. (4b) for dimensionality d ≤ 4,
which leads to the exact exponents ν(d = 1) = 1, ν(d = 2) = 3/4, and ν(d = 3) ≈ 10/17 ≈ 0.588 and exact limiting behavior upon approaching the critical dimension d = 4, above which ν = 1/2. In general, this scaling form holds for any measure of polymer size and any polymer topology in the linear polymer universality class (linear, ring, comb, star, etc.) where the constant b is polymer and topology specific. We note that the large zg asymptotic scaling of α(zg) predicted by Eq. (4a) coincides with the scaling arguments given by Zrinyi and Horkay [20] for the large repulsive excluded volume interaction regime where α(zg) ~ zg 1/5 for zg → ∞.
Swelling factor α for PVAc-isopropyl alcohol gels as a function of the excluded volume interaction zg in the good solvent regime (zg > 0). Symbols are the same as in Fig. 1a. Continuous line is a fit of the data to RG expression for α in Eq. (4b) with the gel excluded volume variable zg defined by Zrinyi and Horkay [20]. Inset a: Fit of the swelling data to the tanh function given in Eq. (5). The continuous line through the data points are least squares fit to the phenomenological function [29], α = 1 + B tanh (c zg) with B = 0.4 and c = 3.16. Inset b: Log–log representation of α as a function of (−zg) for PVAc/isopropyl alcohol gels in the deswelling regime below Θ (zg < 0). Zrinyi and Horkay [20] predict that α(zg)3 ~ 1/(−zg) and we compare the data with this scaling relation [solid line in figure: α = 0.31 [1/(−zg)0.33]
In Fig. 2, we also compare our α(zg) data to the simple sigmoidal function,
suggested as an approximation by Karim et al. [29] for describing the swelling and deswelling of both PVAc gels and end-grafted polymer brush layers. In this equation, B and c are phenomenological constants. This functional form was suggested heuristically by the common sigmoidal functional form describing the density profile of interfaces [29], and we show a comparison to this heuristically deduced functional form in inset a of Fig. 2. This form fits also the experimental data reasonably well. Evidently, measurements in the range of a much larger excluded volume interaction strength zg are required to test the scaling prediction of Eq. (5) and the simplicity of the tanh approximant of α(zg) for practical applications.
At any rate, the resulting swelling curve for our solution cross-linked gels appears to be remarkably universal, at least for this particular type of polymer, and again we observe a close similarity with swelling data observed for the radius of gyration (Rg) of flexible polymer chains in solution [25, 30, 31] when the temperature is varied over a broad range.
The RG method is unfortunately not applicable in the regime in which zg is negative [32, 33] where polymer chains tend to collapse forming a compact globular structure. Since there is no RG treatment of self-attracting polymers, there is no RG theory describing the phase behavior of polymer solutions. Moreover, the assumption of the existence of universality in the scaling functions is more questionable in the regime of polymer deswelling because of the emergence of relevant many-body interactions when the chains start to collapse. [33] Nonetheless, it is plausible to hypothesize that the definition of zg, which accounts for ternary interactions, might be sufficient in the self-attracting polymer regime. Along this lines, Zrinyi and Horkay [20] argued that α(zg)3 should scale in the self-attracting regime as, α(zg)3 ~ 1/(−zg) similar to scaling arguments made previously for linear polymers in solution. In Fig. 2, inset b indicates that this scaling relation indeed describes the PVAc gel behavior in the poor solvent regime. As noted above, the universality of this scaling must be checked through measurements on other solution cross-linked networks.
Influence of solvent quality on the elastic modulus of PVAc gels
We now focus on the effect of excluded volume interaction and varying the cross-link density on the shear modulus G of our solution cross-linked PVAc/isopropyl alcohol gels. Figure 3 shows that G / RT scales as a power of φe. Above the theta temperature, the experimental value of the scaling exponent n is close to the predicted good solvent value 2.25, in agreement with the self-avoiding linear polymer value (Flory estimate of ν ≈ 3/5) and at the theta temperature the scaling exponent (n = 3.1) is near to the theoretical value n = 3. (This is an important test of internal consistency of our hypothesis that our networks should exhibit the scaling properties of linear flexible chains and of our estimation of the theta point from the vanishing of the second osmotic virial coefficient.) The dashed lines in Fig. 3 show the dependence of G/RT on φe when T is varied for identical networks of fixed values of cross-linking density and initial polymer concentration. In particular, the log–log plot indicates that G / RT scales as φe1/3, as one would expect from the classical rubber elasticity theory [3].
Shear modulus G/RT vs polymer concentration φe under equilibrium conditions for PVAc/isopropyl alcohol gels at different T. Dashed lines: dependence of G/RT for nominally identical gel samples on φe. Inset shows the variation of the power-law exponents with T. Symbols are the same as in Fig. 1a
We note that G/RT extrapolates to a common value corresponding to the material in its dried state, φ = 1. Neither the variation of the polymer concentration at cross-linking nor the length of the network chains affect this value.
Figure 4 shows the T dependence of G in terms of similar reduced variables as that of network swelling and deswelling in the previous section. The symbols designating the samples and the thermodynamic conditions are the same as the network swelling data described above. As a first stage of the data reduction, the inset to Fig. 4 shows G/RT as a function of the reduced temperature, τ = (T–Θ)/T [19]. The main figure shows that all our G observations can described by a near universal function of zg, where this scaling applies in both the good and poor solvent regimes. This data reduction would appear to indicate that our equation of state description of the swelling behavior of the PVAc gels also applies to G. Further studies are required to test the universality of this reduced variable scaling for G for other solution cross-linked polymer networks having different chemistries and solvents. The shear modulus of these solution cross-linked gels evidently progressively diminishes with increased gel swelling over the observed range of network swelling.
Shear modulus G normalized by its value at the theta temperature Gθ as a function of the dimensionless excluded volume interaction parameter zg for PVAc gels swollen in isopropyl alcohol. Symbols are the same as in Fig. 1a. Inset: Shear modulus G/RT as a function of τ for PVAc/isopropyl alcohol gels
Recently, it has been observed that the elasticity of polymer networks is influenced by enthalpic interactions [34] in addition to purely entropic changes with network deformation, but no theoretical explanation of this phenomenon was presented. It is evident that the dependence of G on zg implies that this quantity is enthalpy dependent in a specific fashion.
Conclusions
The swelling of polymer gels formed by cross-linking polymers in solutions is discussed under variable solvent quality conditions. As opposed to previous studies, the theta condition is used as a reference point for describing network swelling. The reduced variable description of network swelling relies on the reduced excluded volume interaction parameter zg appropriate to network polymers. The reduced variable description of the effect of solvent quality on swelling of polymer networks is extended to describe the solvent quality dependence of the shear modulus of solution cross-linked polymer gels.
We note that further experimental studies on well-characterized networks will be required to better understand the material limitations of the scaling description described in the present work, and specifically the universality of the network dimensions on the excluded volume interaction zg below the theta point.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
A.S. Hoffman, Adv. Drug. Deliv. 64, 18 (2012)
W. Richtering, B.R. Saunders, Soft Matter 10, 3695 (2014)
H.M. James, E. Guth, J. Chem. Phys. 11, 455 (1943)
F.T. Wall, P.J. Flory, J. Chem. Phys. 19, 143 (1951)
P.J. Flory, J.J. Rehner, J. Chem. Phys. 11, 512 (1943)
P.J. Flory, J.J. Rehner, J. Chem. Phys. 11, 521 (1943)
T.S. Lin, R. Wang, J.A. Johnson, B.D. Olsen, Macromolecules 52, 1685 (2019)
N.R. Richbourg, N.A. Peppas, Progr. Polym. Sci. 105, 101243 (2020)
P.G. de Gennes, Scaling Concepts in Polymer Physics (Cornell University Press, Ithaca, 1979)
P.J. Flory, Principles of Polymer Chemistry (Cornell University Press, Ithaca, 1953)
P.J. Flory, Discuss. Faraday Soc. 49, 7 (1970)
T. Yasuda, N. Sakumichi, U. Chung, T. Sakai, Phys. Rev. Lett. 125, 267801 (2020)
K. Dusek, M.D. Smrckova, Gels 6, 22 (2020)
C. Wang, T. Hashimoto, Y. Chuang, K. Tanaka, Y. Chang, T. Yang, M. Huang, Macromolecules 55, 9152 (2022)
F. Horkay, M. Zrinyi, Macromolecules 15, 1306 (1982)
A. Chremos, J.F. Douglas, P.J. Basser, F. Horkay, Soft Matter 18, 6278 (2022)
G. Parisi, N. Sourlas, Phys. Rev. Lett. 46, 871 (1981)
M. Shibayama, T. Tanaka, J. Chem. Phys. 97, 6829 (1992)
M. Zrinyi, F. Horkay, Macromolecules 17, 2805 (1984)
M. Zrinyi, F. Horkay, Macromolecules 22, 394 (1989)
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure accurately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
M. Mooney, J. Appl. Phys. 11, 582 (1940)
R.S. Rivlin, Phil. Trans. R. Soc. A 241, 379 (1948)
F. Horkay, J.F. Douglas, Gels 8, 96 (2022)
H. Yamakawa, Modern Theory of Polymer Solutions (Harper and Row, New York, 1971)
K.F. Freed, J.F. Douglas, J. Chem. Phys. 88, 2764 (1988)
J.F. Douglas, J. Roovers, K.F. Freed, Macromolecules 23, 4168 (1990)
J.F. Douglas, K.F. Freed, Macromolecules 17, 2344 (1984)
A. Karim, J.F. Douglas, F. Horkay, L.J. Fetters, S.K. Satija, Physica B 221, 331 (1996)
K.F. Freed, Renormalization Group Theory of Macromolecules (Wiley-Interscienc, New York, 1987)
J.F. Douglas, K.F. Freed, Macromolecules 18, 201 (1985)
J.F. Douglas, Macromolecules 22, 1786 (1989)
J.F. Douglas, B.J. Cherayil, K.F. Freed, Macromolecules 18, 2455 (1985)
Y. Yoshikawa, N. Sakumichi, U. Chung, T. Sakai, Phys. Rev. X 11, 011045 (2021)
Acknowledgments
This research was supported by the Intramural Research Program of the NICHD, NIH.
Funding
This work is based upon activities supported by the Intramural Research Program of the NICHD / National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception: J.F.D., F.H., Methodology: F.H., J.F.D., Experimental work: F.H., Writing: J.F.D., F.H. Data analysis: F.H., J.F.D
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Douglas, J.F., Horkay, F. Influence of solvent quality on the swelling and shear modulus of polymer gels chemically cross-linked in solution. MRS Advances (2024). https://doi.org/10.1557/s43580-024-00829-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43580-024-00829-w