Abstract
Synaptic plasticity refers to activity-dependent synaptic strengthening or weakening between neurons. It is usually associated with homosynaptic plasticity, which refers to a synaptic junction controlled by interactions between specific neurons. Heterosynaptic plasticity, on the other hand, lacks this specificity. It involves much larger populations of synapses and neurons and can be associated with changes in synaptic strength due to nonlocal alterations in the ambient electrochemical environment. This paper presents specific examples demonstrating how variations in the ambient electrochemical environment of lipid membranes can impact the nonlinear dynamical behaviors of memristive and memcapacitive systems in droplet interface bilayers (DIBs). Examples include the use of pH as a modulatory factor that alters the voltage-dependent memristive behavior of alamethicin ion channels in DIB lipid bilayers, and the discovery of long-term potentiation (LTP) in a lipid bilayer-only system after application of electrical stimulation protocols.
Graphical abstract



Copyright 2018 American Chemical Society

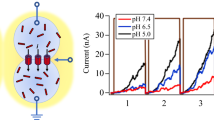
Adapted from MRS Bulletin 48, 13–21 (2023)

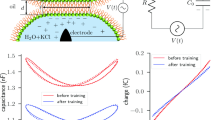
Adapted from Ref. 35, Evidence for long-term potentiation in phospholipid membranes © 2022 by Scott et al. Licensed under CC BY 4.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/

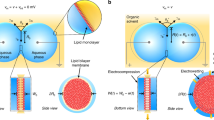
Copyright 2023 American Chemical Society
Similar content being viewed by others
Data availability
Data available from authors upon request.
References
S. Kumar, R.S. Williams, Z. Wang, Third-order nanocircuit elements for neuromorphic engineering. Nature 585, 518–523 (2020). https://doi.org/10.1038/s41586-020-2735-5
J. Tang, F. Yuan, X. Shen, Z. Wang, M. Rao, Y. He, Y. Sun, X. Li, W. Zhang, Y. Li, B. Gao, H. Qian, G. Bi, S. Song, J.J. Yang, H. Wu, Bridging biological and artificial neural networks: fundamentals, progress, and challenges. Adv. Mater. 31, 1902761 (2019). https://doi.org/10.1002/adma.20190276
S. Kumar, X. Wang, J.P. Strachan, Y. Yang, W.D. Lu, Dynamical memristors for higher-complexity neuromorphic computing. Nat. Rev. 7, 575–591 (2022). https://doi.org/10.1038/s41578-022-00434-z
D.O. Hebb, The organization of behavior: a neuropsychological theory (Wiley, New York, 1949)
C.H. Bailey, M. Giustetto, Y.Y. Huang, R.D. Hawkins, E.R. Kandel, Is heterosynaptic plasticity essential for stabilizing Hebbian plasticity and memory? Nat. Rev. Neurosci. 1, 11–20 (2000). https://doi.org/10.1038/35036191
Y. Wang, J. Yang, Z. Wang, J. Chen, Q. Yang, Z. Lu, Y. Zhou, Y. Zhai, Z. Li, S.-T. Han, Near-infrared annihilation of conductive filaments in quasiplane MoSe2/Bi2Se3 nanosheets for mimicking heterosynaptic plasticity. Small 15, 1805431 (2019). https://doi.org/10.1002/smll.201805431
V.F. Castellucci, H. Blumenfeld, P. Goelet, E.R. Kandel, Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J. Neurobiol. 20, 1–9 (1989). https://doi.org/10.1002/neu.480200102
Y. Yang, B. Chen, W.D. Lu, Memristive physically evolving networks enabling the emulation of heterosynaptic plasticity. Adv. Mater. 27, 7720–7727 (2015). https://doi.org/10.1002/adma.201503202
Y. Li, E.J. Fuller, J.D. Sugar, S. Yoo, D.S. Ashby, C.H. Bennett, R.D. Horton, M.S. Bartsch, M.J. Marinella, W.D. Lu, A.A. Talin, Filament-free bulk resistive memory enables deterministic analogue switching. Adv. Mater. 32, 2003984 (2020). https://doi.org/10.1002/adma.202003984
C. Mead, Analog VLSI and neural systems (Addison-Wesley, Boston, 1989)
Y. He, L. Zhu, Y. Zhu, C. Chen, S. Jiang, R. Liu, Y. Shi, Q. Wan, Recent progress on emerging transistor-based neuromorphic devices. Adv. Intell. Syst. 3, 2000210 (2021). https://doi.org/10.1002/aisy.202000210
J. Jiang, T. Xu, J. Lu, L. Sun, Z. Ni, Defect engineering in 2D materials: precise manipulation and improved functionalities. Research 2019, 4641739 (2019). https://doi.org/10.34133/2019/4641739
F. Liu, Z. Fan, Defect engineering of two-dimensional materials for advanced energy conversion and storage. Chem. Soc. Rev. 52, 1723–1772 (2023). https://doi.org/10.1039/D2CS00931E
M. Tripathi, F. Lee, A. Michail, D. Anestopoulos, J.G. McHugh, S.P. Ogilvie, M.J. Large, A.A. Graf, P.J. Lynch, J. Parthenios, K. Papagelis, S. Roy, M.A.S.R. Saadi, M.M. Rahman, N.M. Pugno, A.A.K. King, P.M. Ajayan, A.B. Dalton, Structural defects modulate electronic and nanomechanical properties of 2D materials. ACS Nano 15, 2520–2531 (2021). https://doi.org/10.1021/acsnano.0c06701
J.S. Najem, G.J. Taylor, R.J. Weiss, M.S. Hasan, G. Rose, C.D. Schuman, A. Belianinov, C.P. Collier, S.A. Sarles, Memristive ion channel-doped biomembranes as synaptic mimics. ACS Nano 12, 4702–4711 (2018). https://doi.org/10.1021/acsnano.8b01282
J.S. Najem, M.S. Hasan, R.S. Williams, R.J. Weiss, G.S. Rose, G.J. Taylor, S.A. Sarles, C.P. Collier, Dynamical nonlinear memory capacitance in biomimetic membranes. Nat. Commun. 10, 3239 (2019). https://doi.org/10.1038/s41467-019-11223-8
H. Bayley, B. Cronin, A. Heron, M.A. Holden, W.L. Hwang, R. Syeda, J. Thompson, M. Wallace, Droplet interface bilayers. Mol. Biosyst. 4, 1191–1208 (2008). https://doi.org/10.1039/B808893D
M.A. Holden, D. Needham, H. Bayley, Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc. 129, 8650–8655 (2007). https://doi.org/10.1021/ja072292a
G. Villar, A.D. Graham, H. Bayley, A tissue-like printed material. Science 340, 48–52 (2013). https://doi.org/10.1126/science.1229495
R. Kawano, Y. Tsuji, K. Kamiya, T. Kodama, T. Osaki, N. Miki, S. Takeuchi, A portable lipid bilayer system for environmental sensing with a transmembrane protein. PLoS ONE 9, e102427 (2014). https://doi.org/10.1371/journal.pone.0102427
G. Taylor, M.A. Nguyen, S. Koner, E. Freeman, C.P. Collier, S.A. Sarles, Electrophysiological interrogation of asymmetric droplet interface bilayers reveals surface-bound alamethicin induces lipid flip-flop. Biochim. Biophys. Acta Biomembr. 1861, 335–343 (2019). https://doi.org/10.1016/j.bbamem.2018.07.001
J.B. Boreyko, P. Mruetusatorn, S.A. Sarles, S.T. Retterer, C.P. Collier, Evaporation-induced buckling and fission of microscale droplet interface bilayers. J. Am. Chem. Soc. 135, 5545–5548 (2013). https://doi.org/10.1021/ja4019435
L. Chua, Memristor—the missing circuit element. IEEE Trans. Circuit Theory 18, 507–519 (1971). https://doi.org/10.1109/TCT.1971.1083337
M. Montal, P. Mueller, Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. U.S.A. 69, 3561–3566 (1972). https://doi.org/10.1073/pnas.69.12.3561
F. Szoka, D. Papahadjopoulos, Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 9, 467–508 (1980). https://doi.org/10.1146/annurev.bb.09.060180.002343
T.R. Heimburg, The capacitance and electromechanical coupling of lipid membranes close to transitions: the effect of electrostriction. Biophys. J. 103, 918–929 (2012). https://doi.org/10.1016/j.bpj.2012.07.010
W.T. McClintic, H.L. Scott, N. Moore, M. Farahat, M. Maxwell, C.D. Schuman, D. Bolmatov, F.N. Barrera, J. Katsaras, C.P. Collier, Heterosynaptic plasticity in biomembrane memristors controlled by pH. MRS Bull. 48, 13–21 (2023). https://doi.org/10.1557/s43577-022-00344-z
R.S. Zucker, W.G. Regehr, Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002). https://doi.org/10.1146/annurev.physiol.64.092501.114547
H. Markram, M. Tsodyks, Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382, 807–810 (1996). https://doi.org/10.1038/382807a0
Z. Wang, S. Joshi, S.E. Savel’ev, H. Jiang, R. Midya, P. Lin, M. Hu, N. Ge, J.P. Strachan, Z. Li, Q. Wu, M. Barnell, G.-L. Li, H.L. Xin, R.S. Williams, Q. Xia, J.J. Yang, Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 16, 101–108 (2017). https://doi.org/10.1038/nmat4756
R.S. Cantor, The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids 101, 45–56 (1999). https://doi.org/10.1016/s0009-3084(99)00054-7
H.L. Scott, J.M. Westerfield, F.N. Barrera, Determination of the membrane translocation pK of the pH-Low Insertion Peptide. Biophys. J. 113, 869–879 (2017). https://doi.org/10.1016/j.bpj.2017.06.065
P.-Y. Deng, V.A. Klyachko, The diverse functions of short-term synaptic plasticity components in synaptic computations. Commun. Integr. Biol. 4, 543–548 (2011). https://doi.org/10.4161/cib.4.5.15870
T.V. Bliss, A.R. Gardner-Medwin, Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J. Physiol. 232, 357–374 (1973). https://doi.org/10.1113/jphysiol.1973.sp010274
H.L. Scott, D. Bolmatov, P.T. Podar, Z. Liu, J.J. Kinnun, B. Doughty, R. Lydic, R.L. Sacci, C.P. Collier, J. Katsaras, Evidence for long-term potentiation in phospholipid membranes. Proc. Natl. Acad. Sci. U.S.A. 119, e2212195119 (2022). https://doi.org/10.1073/pnas.2212195119
W.C. Clapp, J.P. Hamm, I.J. Kirk, T.J. Teyler, Translating long-term potentiation from animals to humans: a novel method for noninvasive assessment of cortical plasticity. Biol. Psychiatry 71, 496–502 (2012). https://doi.org/10.1016/j.biopsych.2011.08.021
R.L. Sacci, H.L. Scott, Z. Liu, D. Bolmatov, B. Doughty, J. Katsaras, C.P. Collier, Disentangling memristive and memcapacitive effects in droplet interface bilayers using dynamic impedance spectroscopy. Adv. Electron. Mater. 8, 2200121 (2022). https://doi.org/10.1002/aelm.202200121
H.L. Scott, D. Bolmatov, U.I. Premadasa, B. Doughty, J.-M.Y. Carrillo, R.L. Sacci, M. Lavrentovich, J. Katsaras, C.P. Collier, Cations control lipid bilayer membrane memcapacitance associated with long-term potentiation. ACS Appl. Mater. Interfaces 15, 44533–44540 (2023). https://doi.org/10.1021/acsami.3c09056
R.J. Clarke, C. Lüpfert, Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: a basis for the Hofmeister effect. Biophys. J. 76, 2614–2624 (1999). https://doi.org/10.1016/S0006-3495(99)77414-X
R.J. Clarke, The dipole potential of phospholipid membranes and methods for detection. Adv. Colloid Interface Sci. 89–90, 263–281 (2001). https://doi.org/10.1016/s0001-8686(00)00061-0
E. Deplazes, B.D. Tafalla, C.G. Cranfeld, A. Garcia, Role of ion-phospholipid interactions in zwitterionic phospholipid bilayer ion permeation. J. Phys. Chem. Lett. 11, 6353–6358 (2020). https://doi.org/10.1021/acs.jpclett.0c01479
G.J. Amador, D. van Dijk, R. Kieffer, D. Tam, Hydrodynamic shear dissipation and transmission in lipid bilayers. Proc. Natl. Acad. Sci. U.S.A. 118, e2100156118 (2021). https://doi.org/10.1073/pnas.2100156118
R.W. Taylor, F. Benz, D.O. Sigle, R.W. Bowman, P. Bao, J.S. Roth, G.R. Heath, S.D. Evans, J.J. Baumberg, Watching individual molecules flex within lipid membranes using SERS. Sci. Rep. 4, 5940 (2014). https://doi.org/10.1038/srep05940
M.M. Lozano, J.S. Hovis, F.R. Moss III., S.G. Boxer, Dynamic reorganization and correlation among lipid raft components. J. Am. Chem. Soc. 138, 9996–10001 (2016). https://doi.org/10.1021/jacs.6b05540
D. Bolmatov, C.P. Collier, D. Zav’yalov, T. Egami, J. Katsaras, Real space and time imaging of collective headgroup dipole motions in zwitterionic lipid bilayers. Membranes 13, 442 (2023). https://doi.org/10.3390/membranes13040442
I. Ermolina, A. Strinkovski, A. Lewis, Y. Feldman, Observation of liquid-crystal-like ferroelectric behavior in a biological membrane. J. Phys. Chem. B 105, 2673–2676 (2001). https://doi.org/10.1021/jp001054y
A.G. Petrov, Electricity and mechanics of biomembrane systems: flexoelectricity in living membranes. Anal. Chim. Acta 568, 70–83 (2006). https://doi.org/10.1016/j.aca.2006.01.108
Acknowledgments
J.K. and C.P.C. are supported through the Scientific User Facilities Division of the Department of Energy (DOE) Office of Science, sponsored by the Basic Energy Science (BES) Program, DOE Office of Science, under Contract No. DE-AC05-00OR22725. D.B. is supported through the National Science Foundation, Division of Molecular and Cellular Biosciences (MCB), under contract No. 2219289. Manuscript preparation was performed at the Center for Nanophase Materials Sciences, a US DOE Office of Science User Facility.
Funding
This study is supported by the Basic Energy Sciences, DE-AC05-00OR22725, to C. Patrick Collier.
Author information
Authors and Affiliations
Contributions
CPC wrote the manuscript. All authors edited and proofread the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bolmatov, D., Katsaras, J. & Patrick Collier, C. Heterosynaptic plasticity in memristive and memcapacitive lipid bilayers: A snapshot review. MRS Advances (2024). https://doi.org/10.1557/s43580-024-00800-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43580-024-00800-9