Abstract
We present the analytic workflow to understand the temperature and illumination-intensity-dependent recombination of the photoinduced charge carriers in organic–inorganic hybrid perovskite solar cells based on methylammonium lead iodide (CH3NH3PbI3) thin films. The temperature-dependent, open-circuit voltage analysis reveals that the recombination of photoinduced charge carriers predominantly occurs at our PEDOT:PSS/CH3NH3PbI3 interface as well as in the depletion region. Taking into account the structural phase transition in CH3NH3PbI3 films, as confirmed using temperature-dependent x-ray diffraction spectra and dielectric constant measurements, the illumination-dependent short-circuit current and open-circuit voltage analysis shows both bimolecular and trap-assisted recombination is dominant in our solution-processed perovskite solar cells.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A swift leap in perovskite solar cells research has resulted in high performance devices with NREL-certified power conversion efficiency (η) of > 25% under simulated one sun,[1] which is higher than that of any other thin film photovoltaic (PV) technology. A considerable amount of research focuses on methylammonium lead iodide (CH3NH3PbI3 or MAPI) benchmark organic–inorganic hybrid perovskite materials.[2,3] Despite the competitive effort in improving PV efficiency, the key challenge is associated with poor materials stability, which is becoming a major obstacle in realizing potentially low-cost and high-efficiency perovskite solar cells.[4] While a majority of perovskite PV work has been done at room temperature or slightly elevated temperature, which is known to be an optimal so-called Goldilocks Environment for the MAPI solar cells,[5] a study of perovskite solar cell operation under a broad range of temperature could reveal fundamental mechanisms behind the temperature dependence of the device performance and stability. Moreover, some specific applications, such as space application during dark–light cycling, involve large temperature variations, which influence the photophysics and photochemistry as well as the charge separation and transport properties in the various materials employed in the device.
In this work, we report the temperature- and illumination intensity-dependent recombination behavior of the photoinduced charge carriers in the p-i-n configuration of perovskite solar cells with PEDOT:PSS as hole transport layer,[6] while most of temperature dependence studies reported so far were done using n-i-p configuration with n-type semiconducting TiO2 as electron transport layer.[7] Perovskite solar cells have been characterized under a wide range of temperature (40–430 K) and illumination light level (1 × 10–3–56.6 mW/cm2). Then, the extracted open-circuit voltage (VOC) and short-circuit current density (JSC) as a function of temperature and illumination intensity were used for the analysis of the recombination of the photoinduced charge carriers. The carrier concentration was estimated via capacitance–voltage (C–V) measurement and drive-level capacitance profiling (DLCP) technique. While the quality of our experimental results is limited by our lab-scale solution processing techniques, our analysis provides insights that are useful for understanding and further developing high-efficiency perovskites PV cells.
Experimental
The methylammonium lead halide (CH3NH3PbI3)-based perovskite solar cell used in this study has the layer sequence (as in [Fig. 1(a)]) of glass/ITO/PEDOT:PSS (35 nm)/CH3NH3PbI3 (320 nm)/C60 (40 nm)/BCP (8 nm)/Al (100 nm) and cross-sectional SEM image (Glass/ITO/PEDOT:PSS/CH3NH3PbI3) is shown in [Fig. 1(b)]. As can be seen from Scanning Electron Microscopy (SEM) image (see [Fig. 1(c)]) of the MAPI film, it is a polycrystalline film with individual crystallite lateral dimensions spread from c.a. 200 to 800 nm. The energy level band diagram of the device structure is shown in [Fig. 1(d)].
The perovskite solar cells were fabricated on pre-patterned ITO-glass substrates. We spin coated approximately 35-nm-thick PEDOT:PSS film on the ITO-glass substrates inside a fume hood and annealed them at 145°C for 20 min in the air. In preparing the perovskite (CH3NH3PbI3) precursor solution, lead iodide (PbI2), methylammonium acetate (CH3NH2∙CH3COOH), and methylammonium iodide (CH3NH3I) were mixed with the molar ratio of 1:1:1. After transferring the PEDOT:PSS coated ITO-glass substrates to a nitrogen filled glovebox, thin films of perovskites (CH3NH3PbI3) were spun on such substrates. Then, we annealed the CH3NH3PbI3 coated substrates at 100°C for 5 min in a humidity (relative humidity: 30–35%) controlled environment outside the glovebox. Finally, to complete the device structure, we sequentially deposited the thin films of C60 (~ 40 nm), BCP (~ 8 nm), and Al electrode (~ 100 nm) using a thermal evaporator unit attached to the N2 glovebox. After completing the device fabrication, solar cells were encapsulated inside a glovebox to ensure no exposure to the environmental moisture and oxygen. Further experimental details and methods of this work can be found in the thesis of the co-author (in the sections 3.2, and 4.4).[8] The overall crystal formation process can be described by the chemical reaction:
It is important to notice that methylammonium acetate decomposition temperature is 97.4°C.[9] Here, following Zhang et al.,[9] we ascribe the role of methylammonium acetate in slowing down of the crystal growth process (crystallization to the perovskite only proceeds as the excess organic component is driven out) thus resulting in a better film uniformity and higher crystal quality. Temperature-dependent X-Ray diffraction (XRD) measurement of perovskite thin films was performed using Rigaku SmartLab X-ray diffractometer equipped with graphite dome heating stage.
Room-temperature perovskite solar cells were characterized using an Oriel class ABA solar simulator and Keithley 2400 source meter. The external quantum efficiency (EQE) was measured using a home-built setup. Under illumination, the temperature-dependent current density–voltage (J–V) characteristics were obtained using a closed-cycle helium (He) cryogenic probe-station in the temperature range from 40 to 430 K. For the J–V characteristics under illumination, we have used a calibrated laser-driven light source (from Energetiq Inc.) possessing illumination intensity 56.6 mW/cm2 in the spectral regime of 400 nm to 850 nm. For the illumination intensity-dependent J–V characteristics, the intensity was varied using a series of calibrated neutral density filters from ~ 1 × 10–3 to 56.6 mW/cm2.
The carrier density was estimated using C–V measurement by varying the frequency from 100 to 106 Hz in dark. The ac-signal amplitude of 30 mVrms was imposed for the C–V measurement, while the dc-bias was varied from − 1.0 to 1.2 V. We also performed DLCP (drive-level capacitance profiling) technique in which the ac-modulation signal varied between 10 and 200 mVrms along with the dc-voltage sweep as in C–V method. The temperature-dependent dielectric constant (κ) of the perovskite absorber was measured using the C–V measurements at zero bias with several frequencies (f) at 1 kHz, 10 kHz, and 100 kHz using a fabricated capacitor structure (e.g., ITO/ CH3NH3PbI3/Al).
Results and discussions
It is known from the literature that MAPI perovskite has three structural phases: (i) orthorhombic (low temperature), (ii) tetragonal, and (iii) cubic (high temperature). Phase transition temperatures for the orthorhombic to tetragonal and for tetragonal to cubic are known to be 162–172 K and 330–338 K, respectively, depending on the isotope composition and heating/cooling cycle.[10] We have used temperature-dependent dielectric constant measurements to estimate the low phase transition temperature in our case to be 160 ± 3 K (heating cycle). See Supporting Information and Fig. S1. We have also obtained the high temperature phase transition from temperature-dependent XRD two-theta scan[11] to be 330 ± 5 K (heating and cooling cycle).
Standard perovskite solar cells used in this study consist of glass/ITO/PEDOT:PSS/CH3NH3PbI3/C60/BCP/Al (see [Fig. 1(a)]). The schematic band diagram vs vacuum of each layer in the stack is shown in [Fig. 1(d)]. When measured at room temperature, the devices exhibit an average efficiency of 13.6% under simulated one sun condition (AM 1.5G, 100 mW/cm2) with a VOC of 819 mV, a fill factor of 66.5%, and JSC of 24.9 mA/cm2. Fig. S2 shows the representative light current–voltage curve and the external quantum efficiency of the device.
Next, we performed J–V sweeps of the device in the temperature range from 40 to 430 K with varying the illumination intensity from 1 × 10–4 to 56.6 mW/cm2 to extract temperature- and illumination intensity-dependent JSC and VOC (see also Fig. S3). To gain some insights into the device operation, we focus on the temperature and illumination-dependent open-circuit voltage (VOC) analysis using a well-established methodology to explore the non-radiative recombination processes.[12] The advantage of focusing on the VOC point in J–V characteristics is that at V = VOC, all photo-generated charge carriers recombine. In addition, this allows negating the effect of series resistance, as there is no net current flow in the device. Further, VOC − T-dependent study predicts the location in the absorber where the majority of generated charge carriers recombine.[13]
Temperature-dependent recombination of the photoinduced charge carriers
At the fixed illumination intensity of 56.6 mW/cm2, the temperature dependence of VOC is shown in Fig. 2—marked with phase transition regimes. It was shown both theoretically and experimentally that VOC vs. T is linear near the room-temperature regime and T = 0 K extrapolation of the linear fit is the measure of the activation energy (\({E}_{a}\)) of the dominant recombination process.[14] The expression of VOC is then given by,
The dependency of VOC on temperature at illumination intensity of 56.6 mW/cm2. The activation energy of dominant recombination is \({E}_{a}\)=1.19 eV which is estimated by extrapolating the VOC data to T = 0 K. The inset shows the UV–Vis spectra of perovskite thin films of thickness 320 nm—show the optical bandgap of 1.53 eV.
where \({J}_{00}\) is the pre-factor for the reverse saturation current (\({J}_{0}\)) which depends on the specific recombination mechanism, and n is the diode ideality factor. Note, the VOC is the measure of quasi-Fermi level separation under illumination and the extrapolated \({E}_{a}\) value is a measure of maximum attainable quasi-Fermi level separation. If \({E}_{a}\) is equals the perovskite absorber bandgap (\({E}_{g}\)) then one would say the recombination is dominated in the semiconductor bulk. In contrary, if \({E}_{a}\) value is lower than \({E}_{g}\) value then it is straightforward to say that majority of generated electrons and holes recombine in the depletion region and PEDOT:PSS/Perovskite interface.[15] For 56.6 mW/cm2 (closest to one Sun illumination we could get on that system), the estimated recombination activation energy (\({E}_{a}\)) is 1.19 eV.
The optical bandgap of CH3NH3PbI3 film is estimated to be 1.53 eV via UV–Vis absorption spectroscopy—as shown in the inset of Fig. 2. Estimated \({E}_{a}\) of 1.19 eV is much lower than the bandgap of perovskite absorber. Large difference in the estimated Ea and the Eg indicates that the most generated carriers recombine at the PEDOT:PSS/perovskite interface and the improvement of material quality as well as the interface properties is required which could lead to even higher efficiency than that of our device (η ~ 13.6%) utilized in this report. Note that the VOC − T plot in Fig. 2 is at 56.6 mW/cm2 and not under one sun illumination (100 mW/cm2). So, one should expect higher \({E}_{a}\) value and recombination in perovskite bulk. However, the VOC − T plots at several intensity (as in Fig. S4) show \({E}_{a}\) varies from 1.11 to 1.19 eV in the intensity range between 0.02 and 56.6 mW/cm2. Although we do no not have full one sun in our characterization (Little higher \({E}_{a}\) value is expected), the slow variation of \({E}_{a}\) with intensity suggests no significant bulk recombination.
Under illumination, most of the charge carriers generated near the top surface of perovskite absorber. So, the recombination dominantly occurs at PEDOT:PSS/perovskite interface as well as in the depletion region. In the case of charge carrier transport, PEDOT:PSS and perovskite (CH3NH3PbI3) film quality and PEDOT:PSS/CH3NH3PbI3 interface quality play significant role. In our PV device, both PEDOT:PSS and CH3NH3PbI3 films were fabricated via solution process technique. In solution-processed thin film deposition technique, it is highly possible to have defective film surface and poor grain growth that leads to grain boundaries.[16] Interface defects and defects at the grain boundaries trap charge carrier and result in open-circuit voltage loss and special passivation schema required to boost the device performance.[17,18] Further, device degradation could be another factor for low \({E}_{a}\) value. Khadka et al. investigated a comparative analysis with encapsulated perovskite solar cells with and without degradation where they found 0.98 and 1.4 eV recombination activation energy, respectively.[14] Our fabricated cell is also encapsulated cell and may have minimal degradation. After device fabrication and preliminary test, we kept our sample in a glovebox environment for about 2 weeks and the experimental data were taken after that and because of that we do not expect much degradation in our device because of age but cannot be neglected. There is another possibility of degradation in our device is the low temperature effect and the instability in the orthorhombic phase.[19] Intentionally, we entered in this phase to explore the device performance in a wide temperature regime. However, in tetragonal phase of the perovskite (during heating) cell, we have not seen almost any changes in device parameters like JSC and VOC—confirming that our device shows minimal degradation. Along with the PEDOT:PSS/CH3NH3PbI3 interface quality, the interface quality between CH3NH3PbI3 and electron transport layer (ETL) C60 (thermally evaporated thin film under high vacuum condition) is also important because of possible electron trapping at the interface.[20] Tress et al. observed the impact of surface states and carrier trapping in perovskite/ETL interface through the observance of non-constant \({E}_{a}\) with light intensity.[21] In our temperature-dependent open-circuit plots at different light level (as in Fig. S4), we observe a slow increase in \({E}_{a}\) with the increase of illumination intensity—dictates the possibility of surface defects.
Illumination-dependent recombination of the photoinduced charge carriers
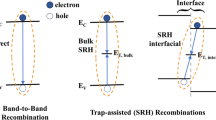
There are three major types of recombination: trap-assisted or defect mediated recombination (Shockley–Read–Hall; SRH type),[22] bimolecular, and monomolecular recombination.[23] The bimolecular recombination refers to the recombination of free electrons and holes in the photoactive layer, while the monomolecular recombination refers to a first-order process including the geminate recombination of a bound electron–hole pair before dissociation, and the SRH recombination at the shallow traps created by defects and impurities in the interfacial layer.[24] Presiding analysis uncovers the types of recombination in the device utilized in this report. So, further we first focus on the illumination-intensity (mW/cm2)-dependent JSC. In general, JSC and intensity (\({I}_{0}\)) follows power law relationship, i.e., \({J}_{SC }\sim {{I}_{0}}^{\alpha }\) and \(\alpha\) value predicts the types of recombination. The power exponent expected to be \(\alpha\) ~1 if first-order monomolecular (either trap-assisted or geminate) recombination is dominating. On the other hand, if the second-order bimolecular recombination process plays a major role, then we should observe the saturation of JSC with the intensity and power exponent should approach a lower value. Figure 3(a) represents the JSC vs. intensity plots (on double-logarithmic scale) in which we see JSC is not linear with the intensity (instead we have two regimes below and above intensity of 0.12 mW/cm2) and tending to saturate at high intensity. We explore both the lower and higher illumination intensity regime. Figure 3(b and c) represents double-logarithmic representation of JSC and intensity in the lower and higher intensity regions, respectively. This result also predicts that we have domination by bimolecular recombination. Considering lower and higher intensity regime, we linearly fit the JSC vs. Intensity data (T: 50–400 K, ΔT = 50 K) as shown in [Fig. 3(b and c)], respectively. The dependency of the slope (α) on temperature (T) is shown in Fig. S5. In the low intensity regime (< 0.12 mW/cm2), the exponent (α) varies between 1 and 1.2—dictating trap-assisted recombination. On the other hand, in the intensity regime (> 0.12 mW/cm2), α varies between 0.35 and 0.9, and suggests bimolecular recombination. Bimolecular recombination in the higher intensity is possible due to higher generation. Though we are not sure why the slope (α) in two intensity regimes is so different, and for simplicity we consider the average slopes vary between α = 0.7 and α = 1.0.
This is the clear signature of the presence of both bimolecular and trap-assisted recombination process in ITO/PEDOT:PSS/CH3NH3PbI3/C60/BCP/Al. To be noted, at short-circuit condition, there is a developed built-in potential which assists the photo-generated carriers to swept out and hence charge collection at the electrode contacts. Because of this, the intensity-dependent JSC is not sensitive enough to traps of any amount, whereas intensity-dependent VOC analysis is.[25]
In further understanding the recombination losses of photo-generated carriers (in account of the limitation of intensity-dependent JSC analysis—as stated earlier) during device operation, the presentation of VOC as a function of illumination light level is important. The irradiation-dependent VOC analysis is a versatile diagnostic tool to interrogate the influences of traps in the recombination process.[26] The intensity-dependent VOC expression can be written as, \({V}_{OC}=\frac{{k}_{B}T}{q}{\text{ln}}\left({{I}_{0}}^{\xi }\right)+C\). Here, ξ is the exponent (ideality factor), kB is the Boltzmann’s constant, I0 is the intensity of illumination (mW/cm2) light, and C is the constant. Even complex, ideally, the slope of the semi-logarithmic plot of VOC and the illumination intensity should give a slope of \(\frac{{k}_{B}T}{q}\) for the absence of trap-assisted (SRH type) recombination. The deviation of the slope from \(\frac{{k}_{B}T}{q}\) signifies the influence of traps. We present semi-logarithmic plots of VOC (V) vs. intensity (in the range 0.02–56.6 mW/cm2) at various temperatures (T: 50–400 K, ΔT = 50 K) and shown in [Fig. 4(a)]. Note that, we exclude the lower intensities (below 0.02 mW/cm2) for which VOC deviates downward due to shunting effect as discussed before. We extracted the temperature (50–400 K) slopes from the fitting and shown in [Fig. 4(b)]. A slight variation of the slope from \(\frac{{k}_{B}T}{q}\) in all phases suggest that we have mixture of bimolecular and trap-assisted recombination.[27] Also, in the orthorhombic and cubic phase (slope > \(\frac{{k}_{B}T}{q}\)) suggest the high possibility to have higher trap-assisted recombination.
The temperature-dependent VOC analysis at fixed illumination level suggests recombination of majority of generated charge at the PEDOT:PSS/Perovskite interface. Also, the intensity-dependent JSC and VOC analysis suggests the presence of both bimolecular and trap-assisted recombination. In solution-processed perovskite solar cell fabrication, it is highly possible to incorporate chemical impurities which act as defects and locate at the interface. Also, perovskite itself may have some structural disorder and have grain boundaries. All together leads to trap-assisted recombination. As discussed previously, to improve the device performance, the improvement of PEDOT:PSS and CH3NH3PbI3 materials, and the PEDOT:PSS/CH3NH3PbI3 and CH3NH3PbI3/C60 interface qualities are required.[17,18,21] Introduction of higher bandgap materials (Compared to PEDOT:PSS) for hole transport layers (Such as Cu2O,[28] NiOx[29] etc.) in place of PEDOT:PSS leads to enhancement of JSC through higher solar energy absorption and VOC increase due to the reduction of front interface recombination. In addition, an approach of bifacial photovoltaics, harvest solar irradiance from both their front and rear surfaces is a great interest in boosting energy conversion efficiency.[30] Finally, we have used C–V and DLCP methods to estimate the carrier concentration in MAPI absorber via carrier density profiling (see Supporting Information, Figs. S6 and S7) of 3.7 × 1016 cm−3.
Conclusions
We exploit the wide temperature- and illumination-dependent complete device analysis with an ITO/PEDOT:PSS/CH3NH3PbI3/C60/BCP/Al-based solar cell. The analysis is helpful to understand the phase transition behavior and its impact on the device parameters particularly the JSC and VOC. The temperature-dependent VOC analysis suggests that the majority of generated charge carriers recombine at the PEDOT:PSS/Perovskite interface and in the depletion regime. The combined illumination intensity-dependent analysis of JSC and VOC predicts in the device that we have mixture of bimolecular and trap-assisted interface recombination. We also perform CV and DLCP analysis and estimate the carrier concentration of 3.7 × 1016 cm−3. We estimated the room-temperature dielectric constant of 9.45 and explored its temperature dependency—correlated with the temperature-dependent either JSC or VOC and the phase transition in CH3NH3PbI3-based perovskite. In conclusion, the information extracted in this report is valuable in developing further device technology based on halide perovskite and understanding device physics of the CH3NH3PbI3-based PV cells.
Data availability
All data presented in this manuscript are available from the corresponding author upon reasonable request.
References
M.A. Green, E.D. Dunlop, M. Yoshita, N. Kopidakis, K. Bothe, G. Siefer, X. Hao, Prog. Photovoltaics Res. Appl. 31, 651–663 (2023)
M. De Santos Los, H.J. Cortina-Marrero, M.A. Ruíz-Sánchez, L. Hechavarría-Difur, F.J. Sánchez-Rodríguez, M. Courel, H. Hu, Solar Energy 199, 198–205 (2020)
J. Oh, H. Lee, M.Y. Ryu, Curr. Appl. Phys. 56, 119–125 (2023)
N. Li, X. Niu, Q. Chen, H. Zhou, Chem. Soc. Rev. 49, 8235–8286 (2020)
T.J. Jacobsson, W. Tress, J.P. Correa-Baena, T. Edvinsson, A. Hagfeldt, J. Phys. Chem. C 120, 11382–11393 (2016)
W. Yoon, J. E. Boercker, M. P. Lumb, J. G. Tischler, P. P. Jenkins, R. J. Walters, 2014 IEEE 40th photovoltaic specialist conference, PVSC 2014, pp. 1577–1580 (2014)
M. Raj, A. Kumar, A. Kumar, K. Laref, S. Singh, A. Sharma, Mater. Lett. 313, 131692 (2022)
M. Hasan, thesis, Texas State University, San Marcos (2019)
W. Zhang, M. Saliba, D.T. Moore, S.K. Pathak, M.T. Horantner, T. Stergiopoulos, S.D. Stranks, G.E. Eperon, J.A. Alexander-Webber, A. Abate, A. Sadhanala, S. Yao, Y. Chen, R.H. Friend, L.A. Estroff, U. Wiesner, H.J. Snaith, Nat. Commun. 6, 6142 (2015)
P.S. Whitfield, N. Herron, W.E. Guise, K. Page, Y.Q. Cheng, I. Milas, M.K. Crawford, Sci. Rep. 6, 1–16 (2016)
T.J. Jacobsson, L.J. Schwan, M. Ottosson, A. Hagfeldt, T. Edvinsson, Inorg. Chem. 54, 10678–10685 (2015)
V. Sarritzu, N. Sestu, D. Marongiu, X. Chang, S. Masi, A. Rizzo, S. Colella, F. Quochi, M. Saba, A. Mura, G. Bongiovanni, Sci. Rep. 7, 44629 (2017)
D.B. Khadka, Y. Shirai, M. Yanagida, K. Miyano, J. Mater. Chem. C Mater. 6, 162–170 (2018)
D.B. Khadka, Y. Shirai, M. Yanagida, K. Miyano, J. Mater. Chem. C Mater. 6, 162–170 (2017)
S. Paul, S. Grover, I.L. Repins, B.M. Keyes, M.A. Contreras, K. Ramanathan, R. Noufi, Z. Zhao, F. Liao, J.V. Li, IEEE J. Photovolt. 8, 871–878 (2018)
S. Srivastava, S. Ranjan, L. Yadav, T. Sharma, S. Choudhary, D. Agarwal, A. Singh, S. Satapathi, R.K. Gupta, A. Garg, K.S. Nalwa, Commun. Mater. 4, 1–29 (2023)
E. Ochoa-Martinez, M. Ochoa, R.D. Ortuso, P. Ferdowsi, R. Carron, A.N. Tiwari, U. Steiner, M. Saliba, ACS Energy Lett. 6, 2626–2634 (2021)
Y. Qi, M. Almtiri, H. Giri, S. Jha, G. Ma, A.K. Shaik, Q. Zhang, N. Pradhan, X. Gu, N.I. Hammer, D. Patton, C. Scott, Q. Dai, Adv. Energy Mater. 12, 2202713 (2022)
N.-K. Kim, Y.H. Min, S. Noh, E. Cho, G. Jeong, M. Joo, S.-W. Ahn, J.S. Lee, S. Kim, K. Ihm, H. Ahn, Y. Kang, H.-S. Lee, D. Kim, Sci. Rep. 7, 4645 (2017)
A. Said, E. Aydin, E. Ugur, Z. Xu, C. Deger, B. Vishal, A. Vlk, P. Dally, B.K. Yildirim, R. Azmi, J. Liu, E.A. Jackson, H.M. Johnson, M. Gui, H. Richter, A.R. Pininti, H. Bristow, M. Babics, A. Razzaq, T.G. Allen, M. Ledinský, I. Yavuz, B.P. Rand, S. De Wolf, Nat. Commun. 15, 1–10 (2024)
W. Tress, M. Yavari, K. Domanski, P. Yadav, B. Niesen, J.P. Correa Baena, A. Hagfeldt, M. Graetzel, Energy Environ. Sci. 11, 151–165 (2018)
F. Gao, Z. Li, J. Wang, A. Rao, I.A. Howard, A. Abrusci, S. Massip, C.R. McNeill, N.C. Greenham, ACS Nano 8, 3213–3221 (2014)
T. Hahn, S. Tscheuschner, F.-J. Kahle, M. Reichenberger, S. Athanasopoulos, C. Saller, G.C. Bazan, T.-Q. Nguyen, P. Strohriegl, H. Bässler, A. Köhler, Adv. Funct. Mater. 27, 1604906 (2017)
H. Li, K. Cao, J. Cui, S. Liu, X. Qiao, Y. Shen, M. Wang, Nanoscale 8, 6379–6385 (2016)
Y. Cheng, H.-W. Li, J. Qing, Q.-D. Yang, Z. Guan, C. Liu, S.H. Cheung, S.K. So, C.-S. Lee, S.-W. Tsang, J. Mater. Chem. A Mater. 4, 12748–12755 (2016)
S. Bi, X. Zhang, L. Qin, R. Wang, J. Zhou, X. Leng, X. Qiu, Y. Zhang, H. Zhou, Z. Tang, Chem. Eur. J. 23, 14650–14657 (2017)
Z. Sun, G. Sitbon, T. Pons, A.A. Bakulin, Z. Chen, Sci. Rep. 5, 10626 (2015)
M. Bidikoudi, E. Kymakis, J. Mater. Chem. C Mater. 7, 13680–13708 (2019)
X. Zheng, Z. Song, Z. Chen, S.S. Bista, P. Gui, N. Shrestha, C. Chen, C. Li, X. Yin, R.A. Awni, H. Lei, C. Tao, R.J. Ellingson, Y. Yan, G. Fang, J. Mater. Chem. C Mater. 8, 1972–1980 (2020)
Q. Jiang, Z. Song, R.C. Bramante, P.F. Ndione, R. Tirawat, J.J. Berry, Y. Yan, K. Zhu, Joule 7, 1543–1555 (2023)
Acknowledgments
This work is dedicated in cherished memory of Prof. Alex Zakhidov, a great scientist, mentor, and friend.
Funding
This material is based upon the work supported by National Science Foundation Grant (#1721884), Petroleum Research Fund of the American Chemical Society (#UNI656095-UNI6), and Office of Naval Research Grant (#W911NF-16-1-0518).
Author information
Authors and Affiliations
Contributions
MH characterized all materials used for the device fabrication, fabricated PV device, and performed preliminary device characterization. SP developed the recombination characterization setup, performed measurements, analyzed data, and wrote the manuscript. AZ supervised the project, and managed funding. All authors contributed to scientific discussions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43579_2024_540_MOESM1_ESM.docx
See the supplementary material for (i) structural phase transitions of the MAPI film using temperature dependent X-ray diffraction spectra and dielectric constant, (ii) dark and light JV characteristics and external quantum efficiency (EQE) vs. wavelength plot of MAPI solar cell, (iii) two dimensional representation of VOC and JSC of the MAPI solar cell as function of temperature and illumination intensity, (iv) recombination activation energy range at different illumination level from VOC-T plot, (v) temperature dependent power exponent (α) in \({J}_{SC}\sim {{I}_{0}}^{\alpha }\) , and (vi) carrier concentration in MAPI film and its depth profile using capacitance-voltage (CV) and drive level capacitance profiling (DLCP) technique. Supplementary file1 (DOCX 1939 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paul, S., Hasan, M. & Zakhidov, A. Temperature- and illumination-dependent recombination of the photoinduced charge carriers in organic–inorganic hybrid perovskite solar cells. MRS Communications 14, 201–207 (2024). https://doi.org/10.1557/s43579-024-00540-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43579-024-00540-9