Abstract
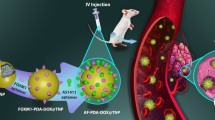
We constructed an iron (Fe2+) metal–organic framework (MOF) in this study. We loaded SN-38 into the MOF before coating the MOF's surface with hyaluronic acid (SN-38@HA@MOF) to improve its ability to target cancer cells. SN-38@HA@MOF can specifically target cancer tissues, has a high loading efficiency, is biocompatible, has a good monodispersity, has a robust cell absorption capacity, and produces significant intracellular reactive oxygen species levels. The in vitro cytotoxicity of the nanocomposites was investigated in different neuroblastoma cancer cells (SH-SY5Y and IMR 32). Even at a 200 µg/mL dosage, the IC50 range was as high as 85%, indicating that MOF and HA@MOF are non-cytotoxic to non-cancerous cells and have outstanding biocompatibility and low toxicity. Flow cytometric analyses investigated the cell death morphological investigation and mechanism. The adaptive multifunctional SN-38@HA@MOF has the potential to treat targeted neuroblastoma cancer.
Graphical abstract










Similar content being viewed by others
Data availability
Data will be made available on request.
Change history
16 February 2024
A Correction to this paper has been published: https://doi.org/10.1557/s43578-024-01300-0
References
A.E. Evans, G.J. D’angio, K. Propert, J. Anderson, H.L. Hann, Prognostic factors in neuroblastoma. Cancer 59, 1853–1859 (1987)
S.B. Whittle, V. Smith, E. Doherty, S. Zhao, S. McCarty, P.E. Zage, Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 17, 369–386 (2017)
J.R. Park, A. Eggert, H. Caron, Neuroblastoma: biology, prognosis, and treatment. Hematol. Oncol. Clin. North Am. 24, 65–86 (2010)
J.R. Park, A. Eggert, H. Caron, Neuroblastoma: biology, prognosis, and treatment. Pediatr. Clin. North Am. 55, 97–120 (2008)
G.M. Brodeur, Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer 3, 203–216 (2003)
J.L. Weinstein, H.M. Katzenstein, S.L. Cohn, Advances in the diagnosis and treatment of neuroblastoma. Oncologist 8, 278–292 (2003)
R. Pandey, A. Gruslova, J. Chiou, A.J. Brenner, S. Tiziani, Stable isotope dilution LC-HRMS assay to determine free SN-38, total SN-38, and SN-38G in a tumor xenograft model after intravenous administration of antibody-Drug conjugate (Sacituzumab Govitecan). Anal. Chem. 92, 1260–1267 (2020). https://doi.org/10.1021/acs.analchem.9b04419
T.M. Cardillo, S.V. Govindan, R.M. Sharkey, P. Trisal, R. Arrojo, D. Liu, E.A. Rossi, C.-H. Chang, D.M. Goldenberg, Sacituzumab Govitecan (IMMU-132), an anti-Trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug. Chem. 26, 919–931 (2015). https://doi.org/10.1021/acs.bioconjchem.5b00223
J.A. Naumann, J.C. Widen, L.A. Jonart, M. Ebadi, J. Tang, D.J. Gordon, D.A. Harki, P.M. Gordon, SN-38 Conjugated gold nanoparticles activated by Ewing Sarcoma specific mRNAs exhibit in vitro and in vivo efficacy. Bioconjug. Chem. 29, 1111–1118 (2018). https://doi.org/10.1021/acs.bioconjchem.7b00774
D. Dutta, S.M. Alex, K.N. Bobba, K.K. Maiti, S. Bhuniya, New insight into a cancer theranostic probe: efficient cell-specific delivery of SN-38 guided by biotinylated Poly(vinyl alcohol). ACS Appl. Mater. Interfaces 8, 33430–33438 (2016). https://doi.org/10.1021/acsami.6b10580
G. Xu, C. Shi, D. Guo, L. Wang, Y. Ling, X. Han, J. Luo, Functional-segregated coumarin-containing telodendrimer nanocarriers for efficient delivery of SN-38 for colon cancer treatment. Acta Biomater. 21, 85–98 (2015). https://doi.org/10.1016/j.actbio.2015.04.021
S.-T. Ning, S.-Y. Lee, M.-F. Wei, C.-L. Peng, S.Y.-F. Lin, M.-H. Tsai, P.-C. Lee, Y.-H. Shih, C.-Y. Lin, T.-Y. Luo, M.-J. Shieh, Targeting colorectal cancer stem-like cells with anti-CD133 antibody-conjugated SN-38 nanoparticles. ACS Appl. Mater. Interfaces 8, 17793–17804 (2016). https://doi.org/10.1021/acsami.6b04403
M. Barani, M.R. Hajinezhad, S. Shahraki, S. Mirinejad, M. Razlansari, S. Sargazi, A. Rahdar, A.M. Díez-Pascual, Preparation, characterization, and toxicity assessment of carfilzomib-loaded nickel-based metal-organic framework: Evidence from in-vivo and in-vitro experiments. J. Drug Deliv. Sci. Technol. 81, 104268 (2023). https://doi.org/10.1016/j.jddst.2023.104268
W. Xu, T. Wang, J. Qian, J. Wang, G. Hou, Y. Wang, X. Cui, A. Suo, D. Wu, Fe (II)-hydrazide coordinated all-active metal organic framework for photothermally enhanced tumor penetration and ferroptosis-apoptosis synergistic therapy. Chem. Eng. J. 437, 135311 (2022)
T. Simon-Yarza, A. Mielcarek, P. Couvreur, C. Serre, Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv. Mater. 30, 1707365 (2018)
H. Zhang, J. Zhang, Q. Li, A. Song, H. Tian, J. Wang, Z. Li, Y. Luan, Site-specific MOF-based immunotherapeutic nanoplatforms via synergistic tumor cells-targeted treatment and dendritic cells-targeted immunomodulation. Biomaterials 245, 119983 (2020). https://doi.org/10.1016/j.biomaterials.2020.119983
J. Cao, X. Li, H. Tian, Metal-organic framework (MOF)-based drug delivery. Curr. Med. Chem. 27, 5949–5969 (2020)
K. Suresh, A.J. Matzger, Enhanced drug delivery by dissolution of amorphous drug encapsulated in a water unstable metal–organic framework (MOF). Angew. Chem. Int. Ed. 58, 16790–16794 (2019)
L.-Q. Fu, X.-Y. Chen, M.-H. Cai, X.-H. Tao, Y.-B. Fan, X.-Z. Mou, Surface engineered metal-organic frameworks (MOFs) based novel hybrid systems for effective wound healing: a review of recent developments. Front. Bioeng. Biotechnol. 8, 576348 (2020)
M. Ibrahim, R. Sabouni, G.A. Husseini, Anti-cancer drug delivery using metal organic frameworks (MOFs). Curr. Med. Chem. 24, 193–214 (2017)
S.S. Nadar, L. Vaidya, S. Maurya, V.K. Rathod, Polysaccharide based metal organic frameworks (polysaccharide–MOF): a review. Coord. Chem. Rev. 396, 1–21 (2019)
M. Wu, Y. Yang, Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 29, 1606134 (2017)
C. Chu, J. Deng, Y. Man, Y. Qu, Evaluation of nanohydroxyapaptite (nano-HA) coated epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes. Mater. Sci. Eng. C 78, 258–264 (2017). https://doi.org/10.1016/j.msec.2017.04.069
A.K. Yadav, P. Mishra, S. Jain, P. Mishra, A.K. Mishra, G.P. Agrawal, Preparation and characterization of HA–PEG–PCL intelligent core–corona nanoparticles for delivery of doxorubicin. J. Drug Target. 16, 464–478 (2008)
R.S. Clarke, M.S. Bruderer, K.P. Ha, A.M. Edwards, RexAB is essential for the mutagenic repair of Staphylococcus aureus DNA damage caused by co-trimoxazole. Antimicrob. Agents Chemother. (2019). https://doi.org/10.1128/AAC.00944-19
J. Wang, X. Wang, Y. Cao, T. Huang, D. Song, H. Tao, Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes. Int. J. Mol. Med. 42, 2604–2614 (2018). https://doi.org/10.3892/ijmm.2018.3817
W. Shi, Y. Kong, Y. Su, M.A. Kuss, X. Jiang, X. Li, J. Xie, B. Duan, Tannic acid-inspired, self-healing, and dual stimuli responsive dynamic hydrogel with potent antibacterial and anti-oxidative properties. J. Mater. Chem. B 9, 7182–7195 (2021). https://doi.org/10.1039/D1TB00156F
V. Grover, P. Chopra, M. Mehta, Natural prokaryotic antimicrobial peptide coated titanium discs prevent Staphylococcus auerus growth and biofilm formation – Implications on peri-implant infections. Mater. Today Proceed. (2021). https://doi.org/10.1016/j.matpr.2021.04.383
M. Shakourian, Y. Yamini, M. Safari, Facile magnetization of metal–organic framework TMU-6 for magnetic solid-phase extraction of organophosphorus pesticides in water and rice samples. Talanta 218, 121139 (2020). https://doi.org/10.1016/j.talanta.2020.121139
P. Raju, T. Ramalingam, T. Nooruddin, S. Natarajan, In vitro assessment of antimicrobial, antibiofilm and larvicidal activities of bioactive nickel metal organic framework. J. Drug Deliv. Sci. Technol. 56, 101560 (2020). https://doi.org/10.1016/j.jddst.2020.101560
M.A. Andrés, M. Benzaqui, C. Serre, N. Steunou, I. Gascón, Fabrication of ultrathin MIL-96(Al) films and study of CO2 adsorption/desorption processes using quartz crystal microbalance. J. Colloid Interface Sci. 519, 88–96 (2018). https://doi.org/10.1016/j.jcis.2018.02.058
L. Huang, S. Zhao, F. Fang, T. Xu, M. Lan, J. Zhang, Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 268, 120557 (2021). https://doi.org/10.1016/j.biomaterials.2020.120557
V. Sokolova, G. Nzou, S.B. van der Meer, T. Ruks, M. Heggen, K. Loza, N. Hagemann, F. Murke, B. Giebel, D.M. Hermann, A.J. Atala, M. Epple, Acta Biomater. 111, 349–362 (2020). https://doi.org/10.1016/j.actbio.2020.04.023
C. He, Y. Hu, L. Yin, C. Tang, C. Yin, Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31, 3657–3666 (2010). https://doi.org/10.1016/j.biomaterials.2010.01.065
R. Pilliadugula, J. Haribabu, M.K. Mohamed Subarkhan, C. Echeverria, R. Karvembu, N. Gopalakrishnan, J. Sci.: Adv. Mater. Dev. 6, 351–363 (2021). https://doi.org/10.1016/j.jsamd.2021.03.003
S. Swaminathan, J. Haribabu, M.K. Mohamed Subarkhan, D. Gayathri, N. Balakrishnan, N. Bhuvanesh, C. Echeverria, R. Karvembu, Impact of aliphatic acyl and aromatic thioamide substituents on the anticancer activity of Ru(ii)-p-cymene complexes with acylthiourea ligands—in vitro and in vivo studies. Dalton Trans. 50, 16311–16325 (2021). https://doi.org/10.1039/D1DT02611A
G. Kalaiarasi, M. Mohamed Subarkhan, C.K. Fathima Safwana, S. Sruthi, T. Sathiya Kamatchi, B. Keerthana, S.L. Ashok Kumar, Inorg. Chim. Acta 535, 120863 (2022). https://doi.org/10.1016/j.ica.2022.120863
N. Mohan, M.K. Mohamed Subarkhan, R. Ramesh, Synthesis, antiproliferative activity and apoptosis-promoting effects of arene ruthenium(II) complexes with N O chelating ligands. J. Organometal. Chem. (2018). https://doi.org/10.1016/j.jorganchem.2018.01.022
S. Balaji, M.K. Mohamed Subarkhan, R. Ramesh, H. Wang, D. Semeril, Synthesis and structure of Arene Ru(II) N∧O-chelating complexes in vitro cytotoxicity and cancer cell death mechanism. Organometallics 39, 1366–1375 (2020). https://doi.org/10.1021/acs.organomet.0c00092
S. Klein, M. Kızaloğlu, L. Portilla, H. Park, T. Rejek, J. Hümmer, K. Meyer, R. Hock, L.V.R. Distel, M. Halik, Enhanced in vitro biocompatibility and water dispersibility of magnetite and cobalt ferrite nanoparticles employed as ROS formation enhancer in radiation cancer therapy. Small 14, 1704111 (2018)
A. Arnida, H.G. Malugin, Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: a comparative study of rods and spheres. J. Appl. Toxicol. 30, 212–217 (2010). https://doi.org/10.1002/jat.1486
K. Giriraj, M.S. Mohamed Kasim, K. Balasubramaniam, S.K. Thangavel, J. Venkatesan, S. Suresh, P. Shanmugam, C. Karri, Various coordination modes of new coumarin Schiff bases toward Cobalt (III) ion: Synthesis, spectral characterization, in vitro cytotoxic activity, and investigation of apoptosis. Appl. Organometal. Chem. 36, e6536 (2022). https://doi.org/10.1002/aoc.6536
M.K. Mohamed Subarkhan, L. Ren, B. Xie, C. Chen, Y. Wang, H. Wang, Novel tetranuclear ruthenium(II) arene complexes showing potent cytotoxic and antimetastatic activity as well as low toxicity in vivo. Eur. J. Med. Chem. (2019). https://doi.org/10.1016/j.ejmech.2019.06.061
S. Swaminathan, J. Haribabu, M.K. Mohamed Subarkhan, G. Manonmani, K. Senthilkumar, N. Balakrishnan, N. Bhuvanesh, C. Echeverria, R. Karvembu, Coordination behavior of Acylthiourea Ligands in their Ru(II)–Benzene Complexes─Structures and anticancer activity. Organometallics 41, 1621–1630 (2022). https://doi.org/10.1021/acs.organomet.2c00127
Author information
Authors and Affiliations
Contributions
JD and DL: was involved in conceptualisation, methodology, investigation, and writing the original draft. YW and RC: took part in conceptualisation, methodology, investigation, and writing-reviewing editing. DL and JP: participated in resources and writing-reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was updated to correct the affiliation of author Jindi Peng.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, J., Li, D., Wang, Y. et al. Precise construction of hyaluronic acid (HA)-coated drug-loaded iron oxide metal–organic frameworks: Investigation of cell death mechanism in neuroblastoma carcinoma cells. Journal of Materials Research 39, 489–500 (2024). https://doi.org/10.1557/s43578-023-01244-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01244-x