Abstract
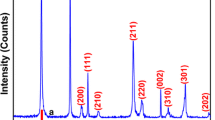
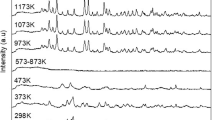
α-MnO2 and γ-MnO2 polymorphs were, respectively, obtained from the plasma precipitation of KMnO4 and Mn(CH3COO)3⋅2H2O precursors. The obtained powders were calcined at 150 °C, 210 °C and 400 °C, and characterized by X-ray diffraction, Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), Thermogravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), nitrogen physisorption and Scanning electron microscopy (SEM). As a result, the calcination does not significantly affect textural properties and crystalline structure of the α-MnO2, while γ-MnO2 is transformed into β-MnO2 for temperatures above 400 °C. The thermal stability α-MnO2 is due to the K+ ions insertion in its 4.6 Å × 4.6 Å tunnels and corroborated the catalytic performance of 100, 98, 98 and 97% compared to 71, 54, 52 and 48% for γ-MnO2 after four successive reuse cycles on Tartrazine Yellow dye. The insertion of cationic species (K+, Na+, Mg2+) into the structure of MnO2 reinforces its crystalline structure and promotes the formation of powerful oxidizing species through oxygen vacant sites.
Graphical Abstract








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
X.F. Guo, G.J. Kim, Synthesis of ordered mesoporous manganese oxides by double replication for use as an electrode material. Bull. Korean Chem. Soc. 32, 186–190 (2011). https://doi.org/10.5012/bkcs.2011.32.1.186
C. Wang, R. Yan, M. Cai, Y. Liu, S. Li, Appl. Surf. Sci. 610, 155346 (2023). https://doi.org/10.1016/j.apsusc.2022.155346
M. Cai, Y. Liu, C. Wang, W. Lin, S. Li, Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway mechanism and toxicity assessment. Sep. Purif. Technol. 304, 122401 (2023). https://doi.org/10.1016/j.seppur.2022.122401
M. Cai, C. Wang, Y. Liu, R. Yan, S. Li, Boosted photocatalytic antibiotic degradation performance of Cd0.5Zn0.5S/carbon dots/Bi2WO6 S-scheme heterojunction with carbon dots as the electron bridge. Sep. Purif. Technol. 300, 121892 (2022). https://doi.org/10.1016/j.seppur.2022.121892
S. Li, M. Cai, Y. Liu, C. Wang, R. Yan, X. Chen, Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder. Mater. 2, 100073 (2023). https://doi.org/10.1016/j.apmate.2022.100073
S. Li, M. Cai, C. Wang, Ta3N5/CdS Core-Shell S-scheme Heterojunction Nanofibers for Efficient Photocatalytic Removal of Antibiotic Tetracycline and Cr(VI): Performance and Mechanism Insights. Adv. Fiber Mater. 5, 994–1007 (2023). https://doi.org/10.1007/s42765-022-00253-5
C. Hongmin, P.K. Chu, J.H. He, T. Hu, M. Yang, Porous magnetic manganese oxide nanostructures: synthesis and their applications in water treatment. J. Colloid Interface Sci. 359, 68–74 (2011). https://doi.org/10.1016/j.jcis.2011.03.089
H.J. Wang, X.Y. Chen, Kinetic analysis and energy efficiency of phenol degradation in a plasma-photocatalysis system. J. Hazard. Mater. 186, 1888–1892 (2011). https://doi.org/10.1016/j.jhazmat.2010.12.088
S. Li, C. Wang, Y. Liu, Y. Liu, M. Cai, W. Zhao, X. Duan, Chem. Eng. J. 455, 140943 (2023). https://doi.org/10.1016/j.cej.2022.140943
J. Dai, S.F.Y. Li, K.S. Siow, Z. Gao, Synthesis and characterization of the hollandite-type MnO2 as a cathode material in lithium batteries. Electrochim. Acta 45, 2211–2217 (2000). https://doi.org/10.1016/S0013-4686(99)00441-7
J. Zhao, Z. Tao, J. Liang, J. Chen, Facile synthesis of γ-MnO2 structures and their applications in rechargeable Li-ion batteries. Cryst. Growth Des. 8(8), 2799–2805 (2008). https://doi.org/10.1021/CG701044B
J. Liu, Y. Son, J. Cai, X. Shen, S. Suib, M. Aindow, Size control, metal substitution, and catalytic application of cryptomelane nanomaterials prepared using cross-linking reagents. Chem. Mater. 16, 276–285 (2004). https://doi.org/10.1021/cm0303989
V. Subramanian, H. Zhu, B. Wie, Alcohol-assisted room temperature synthesis of different nanostructured manganese oxides and their pseudocapacitance properties in neutral electrolyte. Chem. Phys. Lett. 453, 242–249 (2008). https://doi.org/10.1016/j.cplett.2008.01.042
S.H. Kim, S.J. Kim, M.O. Seung, Preparation of layered MnO2 via thermal decomposition of KMnO4 and its electrochemical characterizations. Chem. Mater. 11, 557–563 (1999). https://doi.org/10.1021/CM9801643
K.A.M. Ahmed, H. Peng, K. Wu, K. Huang, Hydrothermal preparation of nanostructured manganese oxides (MnOx) and their electrochemical and photocatalytic properties. Chem. Eng. J. 172, 531–539 (2011). https://doi.org/10.1016/j.cej.2011.05.070
X. Zhang, P. Yu, H. Zhang, D. Zhang, X. Sun, Y. Ma, Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type MnO2 nanosheets for supercapacitor applications. Electrochim. Acta 89, 523–529 (2013). https://doi.org/10.1016/j.electacta.2012.11.089
Y. Li, J. Wang, Y. Zhang, M. Banis, J. Liu, D. Geng, R. Li, X. Sun, Facile controlled synthesis and growth mechanisms of flower-like and tubular MnO2 nanostructures by microwave-assisted hydrothermal method. J. Colloid Interface Sci. 369, 123–128 (2012). https://doi.org/10.1016/j.jcis.2011.12.013
E. Acayanka, D.S. Kuete, G.Y. Kamgang, S. Nzali, S. Laminsi, P.T. Ndifon, Synthesis, characterization and photocatalytic applications of TiO2/SnO2 nanocomposite obtained under non-thermal plasma condition at atmospheric pressure. Plasma Chem. Plasma Process. 36, 799–811 (2016). https://doi.org/10.1007/s11090-016-9699-0
F.W. Boyom-Tatchemo, S. Nzali, G. Kamgang-Youbi, A. Tiya-Djowe, D. Kuete-Saa, E. Acayanka, S. Laminsi, E.M. Gaigneaux, Gliding arc plasma synthesis of MnO2 nanorods for the plasma-catalytic bleaching of azoic Amaranth Red dye. Top. Catal. 60, 962–972 (2017). https://doi.org/10.1007/s11244-017-0761-9
F.W. Boyom-Tatchemo, F. Devred, G. Ndiffo-Yemeli, S. Laminsi, E.M. Gaigneaux, Plasma-induced reactions synthesis of nanosized α-, γ- and δ-MnO2 catalysts for dye degradation. Appl. Catal. B: Environ. 26, 118159 (2020). https://doi.org/10.1016/j.apcatb.2019.118159
R. Kannan, K. Govindan, S. Selvaraj, P. Ravichandiran, S. Vasanthkumar, Birnessite nanorod-mediated decomposition of methylene blue with common oxidants. Appl. Water. Sci. 3, 335–341 (2013). https://doi.org/10.1007/s13201-012-0058-x
A.H. Germeay, R.G. El-Sharkawy, I.A. Mansour, A.B. Zaki, Catalytic activity of polyaniline/MnO2 composites towards the oxidative decolourization of organic dyes. Appl. Catal. B: Environ. 80, 106–115 (2008). https://doi.org/10.1016/j.apcatb.2007.11.014
S. Hamoudi, A. Sayari, K. Belkacemi, L. Bonneviot, F. Larachi, Catalytic wet oxidation of phenol over PtxAg1-xMnO2/CeO2 catalysts. Catal. Today 62, 379–388 (2000). https://doi.org/10.1016/S0920-5861(00)00439-9
S.-L. Chiam, S.-Y. Pung, F.-Y. Yeoh, Recent developments in MnO2-based photocatalysts for organic dye removal: a review. Environ. Sci. Pollut. Res 27, 5759–5778 (2020). https://doi.org/10.1007/s11356-019-07568-8
W. Gong, X. Meng, X. Tang, P. Ji, Core-shell MnO2-SiO2 nanorods for catalyzing the removal of dyes from water. Catalysts 7, 19 (2017). https://doi.org/10.3390/catal7010019
A.M. Hashem, H.M. Abuzeid, D. Mikhailova, H. Ehrenberg, A. Mauger, C.M. Julien, Structural and electrochemical properties of α-MnO2 doped with cobalt. J. Mater. Sci. -Mater. Electron. 47, 2479–2485 (2012). https://doi.org/10.1007/s10853-011-6071-x
F. Teng, S. Santhanagopalan, D.D. Meng, Microstructure control of MnO2/CNT hybrids under in-situ hydrothermal conditions. Solid State Sci. 12, 1677–1682 (2010). https://doi.org/10.1016/j.solidstatesciences.2010.07.026
J. Wu, H. Huang, L. Yu, J. Hu, Controllable hydrothermal synthesis of MnO2 nanostructures. Adv. Mater. Phys. Chem. 3, 201–205 (2013). https://doi.org/10.4236/ampc.2013.33029
D. Portehault, S. Cassaignon, E. Baudrin, J.-P. Jolivet, Morphology control of cryptomelane type MnO2 nanowires by soft chemistry. Growth mechanisms on aqueous medium. Chem. Mater. 19(22), 5410–5417 (2007). https://doi.org/10.1021/cm071654a
A.K. Sinha, M. Pradhan, T. Pal, Morphology evolution of two-dimensional MnO2 nanosheets and their shape transformation to one-dimensional ultralong MnO2 nanowires for robust catalytic activity. J. Phys. Chem. C 117, 23976–23986 (2013). https://doi.org/10.1021/jp403527p
F.W. Boyom-Tatchemo, F. Devred, S. Laminsi, E.M. Gaigneaux, Temporal post-discharge reactions effect on the oxidative catalytic properties of plasma-precipitated α-MnO2 nanorods. Appl. Catal., A 616, 118109 (2021). https://doi.org/10.1016/j.apcata.2021.118109
D. Zheng, S. Sun, W. Fan, H. Yu, C. Fan, G. Cao, Z. Yin, X. Song, One-step preparation of single-crystalline β-MnO2 nanotubes. J. Phys. Chem. B 109, 16439–16443 (2005). https://doi.org/10.1021/jp052370l
C. Ze-hua, H. Ke-Long, L. Su-Qin, W. Hai-Yan, Preparation and characterization of spinel LiMn2O4 nanorods as lithium-ion battery cathodes. Trans. Nonferrous Met. Soc. China 20, 2309–2313 (2010). https://doi.org/10.1016/S1003-6326(10)60646-2
A.K. Thapa, Y. Hidaka, H. Hagiwara, S. Ida, T. Ishihara, Mesoporous β-MnO2 air electrode modified with Pd for rechargeability in lithium-Air battery. J. Electrochem. Soc. 158(12), A1483–A1489 (2011). https://doi.org/10.1149/2.090112jes
M.W. Dose, S.W. Donne, Kinetic analysis of γ-MnO2 thermal treatment. J. Therm. Anal. Calorim. 105, 113–122 (2011). https://doi.org/10.1007/s10973-011-1445-5
J. Luo, H.T. Zhu, H.M. Fan, J.K. Kang, H.L. Shin, G.H. Rao, J.B. Li, Z.M. Du, Z.X. Shen, Synthesis of single-crystal tetragonal α-MnO2 nanotubes. J. Phys. Chem. Lett. 112, 12594–12598 (2008). https://doi.org/10.1021/jp8052967
X. Huang, C. Pan, X. Huang, Preparation and characterization of γ-MnO2/CNTs nanocomposite. Mater. Lett. 61, 934–936 (2007). https://doi.org/10.1016/j.matlet.2006.06.040
Y. Xiong, Y. Xie, Z. Li, C. Wu, Growth of well-aligned γ-MnO2 monocrystalline nanowires through a coordination-polymer-precursor route. Chem. Eur. J. 9(7), 1645–1651 (2008). https://doi.org/10.1002/chem.200390189
S. Saha, A. Pal, Microporous assembly of MnO2 nanosheets for malachite green degradation. Sep. Sci. Technol. 134, 26–36 (2014). https://doi.org/10.1016/j.seppur.2014.07.021
P. Zhang, X. Li, Q. Zhao, S. Liu, Synthesis and optical property of one-dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 6(1), 323 (2011). https://doi.org/10.1186/1556-276X-6-323
M.A. Ahmed-Hashem, Preparation, characterization, and electrochemical performance of γ-MnO2 and LiMn2O4 as cathodes of lithium batteries. Ionics 10, 206–212 (2004). https://doi.org/10.1007/BF02382818
- D. Dias, R. Monteiro, C. Mota-Caetano, A. Pimentel, E. Fortunato, Study of MnO2 coverage on Ta capacitors with high CV powders, Electronics Components, Assemblies, and Materials Association, Symposium, October-November 2007, Barcelona, Spain (2007), Doi: Rui Monteiro.
L. Wang, J. Wang, F. Jia, C. Wang, M. Chen, Nanoporous carbon synthesized coal tar pitch and its capacitive performance. J. Mater. Chem. A 1, 9498–9507 (2013). https://doi.org/10.1039/C3TA10426E
H. Lin, D. Chen, H. Liu, X. Zou, T. Chen, Effect of MnO2 crystalline structure on the catalytic oxidation of formaldehyde. Aerosol Air Qual. Res. 17, 1011–1020 (2017). https://doi.org/10.4209/aaqr.2017.01.0013
L. Li, C. Nan, J. Lu, Q. Peng, Y. Li, Nanotube α-MnO2: High surface area and enhanced lithium battery properties. Chem. Commun. 48, 6945–6947 (2012). https://doi.org/10.1039/C2CC32306K
Y. Zhao, J. Misch, C.-A. Wang, Facile synthesis and characterization of MnO2 nanomaterials as supercapacitor electrode materials. J. Mater. Sci. -Mater. Electron. 27, 5533–5542 (2016). https://doi.org/10.1007/s10854-016-4457-x
P.F. Smith, B.J. Deibert, S. Kaushik, G. Gardner, S. Hwang, H. Wang, J.F. Al-Sharab, E. Garfunkel, L. Fabris, J. Li, G.C. Dismukes, Coordination geometry and oxidation state requirements of corner-sharing MnO6 octahedra for water oxidation catalysis: An investigation of manganite (γ-MnOOH). Catalysis 6, 2089–2099 (2016). https://doi.org/10.1021/acscatal.6b00099
E. Saputra, S. Muhammad, H. Sun, H.M. Ang, M.O. Tadé, S. Wang, Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ. Sci. Technol. 47, 5882–5887 (2013). https://doi.org/10.1021/es400878c
Y. Peng, H. Chang, Y. Dai, J. Li, Structural and surface effect of MnO2 for low temperature selective catalytic reduction of NO with NH3. Procedia Environ. 18, 384–390 (2013). https://doi.org/10.1016/j.proenv.2013.04.051
H. Ying-Ying, W. Zhao-Yin, J. Jun, Rapid low-cost synthesis and enhanced electrochemical properties of mesoporous Mn3O4 nanorods. J. Inorg. Mater. 28(9), 1045–1050 (2013). https://doi.org/10.3724/SP.J.1077.2013.13146
X. Li, T. Fan, Z. Liu, J. Ding, Q. Guo, D. Zhang, Synthesis and hierarchical pore structure of biomorphic manganese oxide derived from woods. J. Eur. Ceram. Soc. 26, 3657–3664 (2006). https://doi.org/10.1016/j.jeurceramsoc.2005.10.015
S. Saha, A. Pal, Microporous assembly of MnO2 nanosheets for malachite green degradation. Sep. Purif. Technol. 134, 26–36 (2014). https://doi.org/10.1016/j.seppur.2014.07.021
L.-T. Tseng, Y. Lu, H.M. Fan, Y. Wang, X. Liu, P. Munroe, S. Li, J. Yi, Magnetic properties in α-MnO2 doped with alkaline elements. Sci. Rep. 5, 9094 (2015). https://doi.org/10.1038/srep09094
J. Qu, L. Shi, C. He, F. Gao, B. Li, Q. Zhou, H. Hu, G. Shao, X. Wang, J. Qui, Highly efficient synthesis of graphene/MnO2 hybrids and their application for ultrafast oxidative decomposition of methylene blue. Carbon 66, 485–492 (2014). https://doi.org/10.1016/j.carbon.2013.09.025
T. Uematsu, Y. Miyamoto, Y. Ogasawara, K. Suzuki, K. Yamaguchi, N. Mizuno, Molybdenum-doped α-MnO2 as an efficient reusable heterogeneous catalyst for aerobic sulfide oxygenation. Catal. Sci. Technol. 6, 222–233 (2016). https://doi.org/10.1039/C5CY01552A
C. Liu, D. Pan, X. Tang, M. Hou, Q. Zhou, J. Zhou, Degradation of Rhodamine B by the α-MnO2/peroxymonosulfate system. Water Air Soil Pollut. 227(92), 1–10 (2016). https://doi.org/10.1007/s11270-016-2782-6
N.A. Fathy, S.E. El-Shafey, O.I. El-Shafey, W.S. Mohamed, Oxidative degradation of RB19 dye by a novel γ-MnO2/MWCNT nanocomposite catalyst with H2O2. J. Environ. Chem. Eng. 1(4), 858–864 (2013). https://doi.org/10.1016/j.jece.2013.07.028
C.M. Cellier, V. Vromman, V. Ruaux, E.M. Gaigneaux, P. Grange, Sulfation mechanism and catalytic behavior of manganese oxide in the oxidation of methanetiol. J. Phys. Chem. B 108, 9989–10001 (2004). https://doi.org/10.1021/jp049158m
C. Cellier, S. Lambert, E.M. Gaigneaux, C. Poleunis, V. Ruaux, P. Eloy, C. Lahousse, P. Bertrand, J.-P. Pirard, P. Grange, Investigation of the preparation an activity of gold catalyst in the total oxidation of n-hexane. Appl. Catal., B (2007). https://doi.org/10.1016/J.APCATB.2006.01.026
C. Liu, J. Wang, L. Xiang, Synthesis and surface characterization of γ-MnO2 nanostructures. J. Nanomater. 4, 389634 (2013). https://doi.org/10.1155/2013/389634
F.A. Caliman, L.C. Apostol, D. Bulgariu, L. Bulgariu, M. Gavrilescu, Study regarding the sorption of erythrosine from aqueous solution onto soil. Environ. Eng. Manag. J. (2009). https://doi.org/10.30638/eemj.2009.196
Acknowledgments
We are grateful to the “Université catholique de Louvain” for scholarship given to F.W. Boyom Tatchemo from the “Coopération au développement” program.
Funding
Université Catholique de Louvain.
Author information
Authors and Affiliations
Contributions
FWBT: Investigation, Conceptualization, Methodology, Data treatment and interpretation, Writing—original draft, Writing—review & editing. FD: XPS characterization, Data treatment and review. EA: Review & editing. GKY: Review & editing. SN: Review & editing. SL: Resources, Supervision. EG: Funding acquisition, Project administration, Conceptualization, Supervision, Data treatment and interpretation, Resources, Writing—review & editing, Validation.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boyom-Tatchemo, F.W., Devred, F., Acayanka, E. et al. Effect of cation insertion on the stability of gliding arc plasma-precipitated mesoporous MnO2 dye bleaching catalysts. Journal of Materials Research 38, 4144–4156 (2023). https://doi.org/10.1557/s43578-023-01129-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01129-z