Abstract
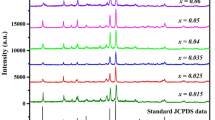
Sodium tetraborate decahydrate was used as boron source, polyethylene glycol was used as the polymerization agent, and nano-Mn3B7O13Cl was prepared by citric acid method with various pH values and mass ratios of citric acid to water. The microstructure, surface morphology, and luminescent properties of the samples were analyzed by XRD, SEM, and fluorescence spectrometer. The results show that the rhombic crystal structure of nano-Mn3B7O13Cl was prepared successfully, and the particle size ranges from 40.3 to 65.7 nm. The smaller the water content and pH, the smaller the particle size and the better the dispersion. At the excitation wavelength of 254 nm, there is a strong emission peak at 429 nm, and the emission intensity is maximum. Under the monitoring wavelength of 564 nm, there is a peak at 460 nm corresponding to a transition of 4T1–6A1, which is the cause of the luminescence of nano-Mn3B7O13Cl, the d–d conversion of Mn2+.
Graphical abstract









Similar content being viewed by others
References
В. Ф. Вeлов, Study on the chambersite Mn3B7O13Cl with nucleon γ ray resonance absorption. Geol. Geochem. 9, 72–78 (1980)
W. Chen, H. Tu, S. Sahi et al., Luminescence of La0.2Y1.8O3 nanostructured scintillators. Opt. Lett. 39(19), 5705–5708 (2014)
S. Thomas, S.N. Rasool, M. Rathaiah et al., Spectroscopic and dielectric studies of Sm3+ ions in lithium zinc borate glasses. J. Non-Cryst. Solids 376(9), 106–116 (2013)
S. Kaur, G. Pal Singh, P. Kaur et al., Cerium luminescence in borate glass and effect of aluminium on blue green emission of cerium ions. J. Lumin. 143(10), 31–37 (2013)
S. Xiong, D. Liang, L. Cao et al., Microstructure and luminescence characteristics of self-doped nano-Mn3B7O13Cl crystal. Mater. Lett. 178, 87–90 (2016)
D. Liang, Study on the Structure, Characteristic and Application of Chambersite and Tungsten-Based Nanomaterial (University of Science and Technology Beijing, Beijing, 2015)
X.N. Shu, L. Cao, C.C. Jia, Effect of nano-chambersite particle size distribution on copper-matrix friction materials. Mater. Sci. Eng. Powder Metall. 20(2), 187–193 (2015)
A. Tomita, T. Sato, K. Tanaka et al., Luminescence channels of manganese-doped spinel. J. Lumin. 109(1), 19–25 (2004)
W.R. Gao, X.M. Wang, W.Q. Xu et al., Luminescent composite polymer fibers: in situ synthesis of silver nanoclusters in electrospun polymer fibers and application. Mater. Sci. Eng. C 42, 333–342 (2014)
R.L. Frost, A. López, R. Scholz et al., Vibrational spectroscopy of the borate mineral Priceite-implications for the molecular structure. Spectrosc. Lett. 48(2), 115–124 (2015)
D. Liang, S. Xiong, Effects of gamma radiation on the microstructure, luminescence and medical properties for nano-Mn3B7O13Cl. Radiat. Eff. Defects Solids 174(7–8), 596–605 (2019)
A.A. Trofimov, Microstructure, luminescence, and performance of garnet polycrystalline ceramic scintillators. Clemson University, 2018.
W. Schnelle, E. Gmelin, O. Crottaz et al., Low temperature specific heat capacity of 3d transition metal chlorine horacites. J. Therm. Anal. Calorim. 56(1), 144–156 (1999)
A.A. Barbosa, S.A. Júnior, Study of a luminescent and antibacterial biomaterial based on hydroxyapatite as support for an antineoplastic drug. J. Mater. Res. 34, 1922–1930 (2019)
W. Simon, M. James et al., Multiple plasmon resonances in small-sized citrate reduced gold nanoparticles. Mater. Chem. Phys. 25(8), 233–238 (2019)
A. Emmanuel, G. Asterios, M. Luca, A mathematical investigation of the Turkevich organizer theory in the citrate method for the synthesis of gold nanoparticles. Chem. Eng. Sci. 7(32), 173–180 (2017)
H. Xuanye, L. Yanan, W. Ping, Effect of improved sol-gel method on composition and micro morphology of Ca3Co4O9. EPJ. Web Conf. 14(1), 140–147 (2017)
A. Sharma, R. Khangarot, P. Misra et al., Band gap reduction and quenching of exchange interaction in sol-gel derived Zn(Al, Cu)O nanostructures. Phys. Scr. 96(7), 115–130 (2021)
F. Umar, P. Ruby, A. Tokeer et al., Electrocatalytic and enhanced photocatalytic applications of sodium niobate nanoparticles developed by citrate precursor route. Sci. Rep. 9(32), 76–90 (2019)
Y. Shengquan, J. Wei, T. Mingjing et al., Fabrication of Nd: YAG transparent ceramics using powders synthesized by citrate sol-gel method. J. Alloys Compd. 23(8), 772–785 (2019)
Acknowledgments
The authors gratefully acknowledge the financial assistance provided by National Natural Science Foundation of China (No. 51904156).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiong, S., Liang, D. Effect of sol process on structure and luminescent properties of nano-Mn3B7O13Cl. Journal of Materials Research 37, 2165–2174 (2022). https://doi.org/10.1557/s43578-022-00499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-022-00499-0