Abstract
Liquid phase (or liquid cell) transmission electron microscopy (TEM) has become a powerful platform for in situ investigation of various chemical processes at the nanometer or atomic level. The electron beam for imaging can also induce perturbation to the chemical processes. Thus, it has been a concern that the observed phenomena in a liquid cell could deviate from the real-world processes. Strategies have been developed to overcome the electron-beam-induced issues. This article provides an overview of the electron-beam effects, and discusses various strategies in liquid cell TEM study of nucleation, growth, and self-assembly of nanoscale materials, where an electron beam is often used to initiate the reactions, and highly electron-beam-sensitive electrochemical reactions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to directly observe the structural, morphological, and compositional changes of materials during chemical reactions has been long-sought. The recent development of liquid phase transmission electron microscopy (TEM) has enabled breakthroughs in characterizing various chemical processes allowing to unveil materials transformation dynamics, and structure and bonding of liquid samples with high spatial resolution.1,2,3 Liquid phase TEM offers unique advantages compared to other in situ characterization methods, although they can be excellent complimentary experiments. For example, in situ x-ray spectroscopy measurements provide ensemble information, but lack spatially resolved individual particle details.4,5 Scanning probe microscopy can only present the surface properties of materials.5,6 Due to the limited spatial resolution, optical microscopy often cannot resolve structural and chemical transformations at the nanoscale, unless chemical tags are used in specific applications.7,8
From the initial development to the current advanced liquid cell TEM, a trajectory of accelerated accomplishments can be found. One indication is the drastically improved spatial resolution, for example, from the initial low magnification (1940s), to a few nanometers (~5 nm) in 2003,9 subnanometer in 2009,10,11 atomic resolution in 2012,12,13,14,15 and beyond16 (Figure 1a). These achievements benefit from the advanced liquid cell design and fabrication. The thin liquid cell development has also enabled chemical analysis using energy-dispersive x-ray spectroscopy (EDS),10,16 which is critical for characterizing many chemical reactions.
Development of liquid phase transmission electron microscopy (LPTEM) for investigating chemical processes. (a) The resolution roadmap of LPTEM with selected milestones (blue), which is overlaid on the original roadmap of TEM created by Pennycook et al.68 (b) A schematic showing effort to connect the LPTEM studies with the real-world chemical processes. ✽: The first closed liquid cell;69 ✩: ~5 nm in 2003;9 ▲: 1 nm—subnanometer in 2009;10,11 △#: atomic resolution in 2012;12,13,14,15 ▽: atomic resolution or beyond.16 ACTEM, aberration-corrected transmission electron microscopy; EDS, energy-dispersive x-ray spectroscopy; HRTEM, high-resolution transmission electron microscopy; EELS, electron energy-loss spectroscopy.
With the improved liquid cell TEM capabilities, a variety of chemical processes have been studied, and discoveries have been made as demonstrated by an outstanding list of publications.10,15,16,17,18,19,20,21 However, many studies utilize the electron beam to drive chemical reactions, thus they are often categorized as electron-beam-driven reactions.22 It has been a concern regarding whether the real-world chemical reaction mechanisms can be informed through liquid cell TEM experiments. To connect the liquid cell TEM observations with the real-world chemical processes (Figure 1b), it is important to understand and control the electron-beam damages.
In this article, we first provide an overview of the electron-beam effects in liquid cell TEM studies. Then, we describe a collection of strategies that have been developed to control or mitigate the electron-beam effects. We depict these efforts through two broad topics of study, such as nucleation, growth, and self-assembly of nanoparticles, and highly electron-beam-sensitive electrochemical reactions.
An overview of electron-beam effects
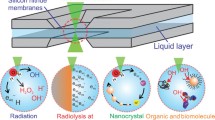
The high-energy electron beam of a transmission electron microscope interacts with a sample can introduce temporary or permanent changes of the materials, besides providing useful information. In conventional (scanning) (S)TEM imaging of samples in vacuum, the electron-beam damages have been attributed to “knock-on,” ionization, electron-beam heating, etc.23 For liquid cell TEM imaging of samples in liquids, the electron-beam effects can be much more complex. Besides the electron-beam damage from the primary electrons, secondary electrons and reactive radicals from radiolysis can further react with the sample or other radiolysis species. These reactions can be dependent on the local environment, such as pH values, composition, diffusion rates/path, gas products. Thus, it is a great challenge to quantify the electron-beam effects in liquid cell TEM experiments, and it is almost impossible to create one formula fit all.
Here, we first highlight some drastic electron-beam-induced phenomena. As shown in Figure 2a, wet PbSe nanocrystals under the electron-beam irradiation quickly aggregated, and their structure and morphology changed dramatically. It was found that the damage mainly results from selective reaction of Se in the nanocrystals under the beam.24 Figure 2b shows a highly converged electron beam can act as a “tweezer” to move Au nanocrystals.25 Another example is the reshaping and splitting of AgCl nanocrystals.26 As shown in Figure 2c, AgCl nanocrystals are negatively charged when reacting with an aqueous solution, thus, the Coulomb force can make the nanocrystal stretched and exploded, thus splitting into smaller nanocrystals. These examples also demonstrate the diverse and complex mechanisms behind the electron-beam effects.
Examples of electron-beam effects, and the studies of electron-beam-induced phenomena. (a) Wet PbSe nanocrystals under the electron-beam irradiation (top), showing structure and morphology compared to those not exposed to an electron beam (bottom). Reprinted with permission from Reference 24. © 2018 American Chemical Society. (b) Electron-beam “tweezer” moving a Au nanocrystal. Reprinted with permission from Reference 25. © 2012 American Chemical Society. (c) Reshaping and splitting of AgCl nanocrystals under electron-beam illumination. Reprinted with permission from Reference 26. © 2018 Wiley. (d) Illustration of radiolysis of water generating many radiolysis species. (e) Calculated concentrations of selected radiolysis species versus time when pure water is irradiated at 7.5 × 107 Gy/s, which corresponds to 300 kV, 1-nA beam current and 1-μm beam radius. Reprinted with permission from Reference 35. © 2014 American Chemical Society. (f) Real-time time-dependent density functional theory calculation of the radiolysis damage of C2H6O2 showed that there is strong competition among different dissociation paths. LUMO, lowest unoccupied molecular orbital; HOMO, highest occupied molecular orbital. Reprinted with permission from Reference 40. © 2019 Royal Society of Chemistry. (g) Growth of Ag nanocrystals showing the morphology of nanocrystals is highly dependent on electron-beam current density. Reprinted with permission from Reference 41. © 2012 American Chemical Society. (h) Growth of Pt-Fe nanowires in an organic solvent in a liquid cell, where the metal ion reduction occurs through the secondary electrons, or the mild reducing agent of oleylamine. Reprinted with permission from References 13 and 43. © 2012 AAAS and © 2013 American Chemical Society.
Various technical approaches have been explored to reduce the electron-beam damage. First, electron-beam effects are often electron dose sensitive, thus reducing the electron dose can lessen the electron-beam damage.22,23 Second, adding radical scavengers into the solution has been reported to mitigate the electron-beam effects. For example, graphene27,28,29 and some ions/molecules30,31,32 have been considered as effective radical scavengers. It is also noted that researchers need to be cautious in exploring radical scavengers, because many factors could play a role in the observed discrepancies in liquid cell TEM experiments. Third, thinner liquid cells could reduce electron inelastic scattering, thus decrease the electron-beam effects, which is an added value to the high-resolution capability. Last, flowing liquid has been proposed to reduce electron-beam effects, on which inconsistent explanations can be found.22,33 One argument is that the liquid flow rate needs to be very fast (meters/second) to carry away the radiolysis products, thus this approach is impractical;22 whereas others emphasize flowing radiolysis scavengers as the mechanisms to reduce electron-beam effects.33
Theoretical calculation of the radiolysis can provide useful guidance for the understanding and controlling of electron-beam effects. Taking the most studied radiolysis of water as an example, there are many radiolysis species and they may react with each other (Figure 2d), thus calculation of the radiolysis species evolution has been mostly empirical. Some simple calculation was conducted to estimate the concentration of each radiolysis species as a function of time (Figure 2e).34,35,36 Because finding the rate constants of each reaction relies on the radiation chemistry literature, only limited systems have been calculated. Besides water, other calculated systems include alcohols and some polar organic solvents.37,38,39 To understand the radiolysis mechanisms of organic molecules, a new systematical procedure based on real-time time-dependent density functional theory (rt-TDDFT) has recently been developed.40 This procedure can describe the ionization cross sections of the electronic states and the fast dissociation processes caused by hot carrier cooling and the Auger decay on deep levels. For the radiolysis damage of C2H6O2, the simulation unexpectedly showed that there is strong competition among different dissociation paths (Figure 2f). As the energy of the incident electron beam changes, the time scales of these dissociation paths and their relative contributions to the molecule damage change significantly. This work has opened many future opportunities to understand the radiolysis mechanisms of different organic molecule degradation under the electron beam. Future theoretical calculation of radiolysis combining with direct experimental measurements of the radiolysis products can be very valuable.
Although electron-beam effects are often not fully understood, an electron beam can be utilized to initiate reactions that enlighten us on the reaction mechanisms relevant to real-world processes. For instance, radicals and electrons from radiolysis of water can be the reducing agent for Ag nanocrystal formation (Figure 2g).41 For the growth of Pt-Fe nanowires in an organic solvent in a liquid cell,13 metal-ion reduction can be achieved by the secondary electrons, or the mild reducing agent of oleylamine (Figure 2h).10,13,42,43 Remarkably, the structure and morphology of nanocrystals obtained in a liquid cell highly resemble those from flask synthesis. The growth of nanocrystals can be controlled and regulated by the fundamental growth mechanisms. To understand the electron-beam effects and connect liquid cell TEM studies of chemical processes with the real-world reactions, strategies have been developed, which are discussed as follows.
Strategies for the study of nucleation, growth, and self-assembly of nanocrystals
An understanding of nanocrystal growth and transformations is crucial to designing synthesis toward targeted nanoparticle morphologies and applications.44,45,46 In liquid phase TEM experiments of nanoparticle growth, using the electron beam to initiate the reactions is convenient for tracking the dynamic processes while imaging.17 Interestingly, although nanoparticle growth kinetics could be strongly influenced by the electron-beam intensity, many studies have demonstrated that the structural and morphological evolution of nanoparticles are directed by the fundamental growth principles, as previously mentioned. Here, we describe various strategies to reveal fundamental growth mechanisms from liquid cell TEM experiments that are useful to designing synthesis of nanoscale materials in the real world.
Collecting more datapoints and large data analysis
A concern regarding the localized information or individual particle events obtained through in situ TEM experiments is whether the observed phenomena are representative, or solely rare events. Further evaluation is often needed to avoid artifacts, or biased conclusions. A straightforward approach, if allowable, is to obtain more datapoints or measuring more particles. The development of advanced electron detector technology has enabled much faster data collection.15,47 In parallel, the modern liquid cell TEM techniques allow better control of reactions and high-resolution imaging. It opens opportunities to tackle new challenging problems that rely on large data collection, and more sophisticated experiments. For example, a systematic study showed multistep nucleation of nanocrystals, where spinodal decomposition into solute-rich and solute-poor liquid phases, nucleation of amorphous nanoclusters within the metal-rich liquid phase, followed by crystallization of these amorphous clusters (Figure 3a).17 In another study, it was found that crystallization of Au nanocrystals at the early stage proceeds through dynamic structural fluctuations between disordered and crystalline states.48
Illustrations of strategies to overcome electron-beam effects in investigating nanocrystal nucleation, growth, and self-assembly, including large data collection and data analysis, implementing complimentary experimental techniques, combining in situ TEM experiments with theoretical calculation, and conducting ex situ control experiments. (a) Analysis of the crystallinities of 74 gold nanoclusters that emerged from gold-rich phases. Crystallinity score versus cluster radii of 74 gold nanoclusters; at least 50 views on each cluster to give a total of 12,229 views. Reprinted with permission from Reference 17. © 2016 Springer Nature. (b) Energy and stability of self-assembly in a liquid cell. The colored circles overlaid on the original image correspond to the total energy of an individual nanoparticle computed from the dipole and van der Waals interaction with nearby particles. Reprinted with permission from Reference 49. © 2018 American Chemical Society. (c) A schematic showing a machine learning model called U-Net neural network. Reprinted with permission from Reference 50. © 2022 American Chemical Society. (d) Size-dependent properties of Pt nanocrystals inferred from their 3D atomic maps, showing radial strain maps of the single-crystalline particles. Strain is indicated by the color scale. Scale bar = 1 nm. Reprinted with permission from Reference 19. © 2020 AAAS. (e, f) Liquid phase transmission electron microscopy (TEM) and “freeze-and-look” TEM study of Hm mesocrystal formation. Reprinted with permission from Reference 56. © 2021 Springer Nature. (e) Ex situ TEM image showing the formation of spindle-shaped Hm mesocrystals from Fh nanoparticles (after 2 h). (f) Liquid phase TEM observation showing Hm nucleation close to the Hm–solution interface, followed by attachment to the seed. (g, h) Liquid phase TEM and Monte Carlo (MC) simulation of the crystallization process in self-assembly of Au nanoplates. Reprinted with permission from Reference 20. © 2019 Springer Nature. (g) Time-lapse TEM images show the real-time crystallization process. Scale bar = 200 nm. (h) MC simulations. (i, j) Two-dimensional growth of cobalt nickel oxide nanosheets with 3D nanoparticles as intermediates. Reprinted with permission from Reference 18. © 2019 Springer Nature. (i) Sequential images showing the formation of cobalt nickel oxide nanosheets through the growth of 3D nanoparticles and 3D-to-2D transformations. Scale bars = 10 nm. (j) Ex situ TEM characterization of the as-obtained cobalt oxide nanosheets under similar experimental conditions in flask synthesis. Reprinted with permission from Reference 17. © 2016 Springer Nature.
With the large data collection, quantitative extraction of parameters from the videos can be the bottlenecked for comprehensive understanding of physical and chemical processes. The image sequences from the videos are often with noise, which adds difficulties to the analysis. The power of computer-aided image analysis has been demonstrated in the early liquid cell TEM experiments. For example, with a custom image analysis method, trajectories of nanoparticle movement and interactions during self-assembly can be obtained with high precision from a stack of noisy images. The computer-aided analysis made it possible to reveal the long-range anisotropic forces driving the formation of nanoparticle patterns during assembly (Figure 3b).49 Nowadays, machine learning models have been able to push the image analysis to a higher quantitative level. As reported recently, a U-Net neural network with high precision and high noise tolerance (Figure 3c) has been developed to resolve nanoparticle diffusion and interaction, reaction kinetics, and assembly dynamics in real time.50 There is no doubt that more and more success will be achieved in machine learning assisted analysis of in situ TEM videos. The ultimate automated data analysis will not be far from the reality.
Incorporating complimentary experimental techniques
In recent years, a variety of powerful electron microscopy techniques have been developed. For example, electron tomography allows 3D atomic imaging of buried dislocation cores in a nanocrystal,51 and deciphering of structural and chemical order/disorder at the atomic level.52,53 For electron microscopy of nanoparticles in liquids with liquid cell TEM, method modifications and innovations have enabled an understanding of the structure and properties of nanocrystals that are inaccessible by other approaches.19,54,55 In Figure 3d, the reconstructed Pt nanoparticles in a liquid cell from electron tomography show particle size-dependent properties, including structural degeneracies, lattice parameter deviations, internal defects, and strain.
Although liquid cell TEM provides a unique opportunity to reveal dynamic processes of nucleation, growth, and self-assembly of nanoparticles, “freeze-and-look” TEM allowing careful examination of materials at frozen states along the transformations can provide value complimentary information. As shown in Figure 3e–f, the combination of in situ liquid cell TEM observation and “freeze-and-look” TEM allow capturing the interface-driven pathways of rarely captured hematite (Hm) mesocrystals in the presence of oxalate (Ox).56
Combining experiments with theoretical calculation
Theoretical calculations have been widely used to support and extend the understanding based on experimental observations through liquid cell TEM. Various theoretical calculation methods have been used. Density functional theory (DFT) calculation is commonly used to rationalize materials transformation pathways. For example, calculation showed that much lower ligand mobility on the {100} facets is responsible for the arresting of {100} growing facets during Pt nanocube formation.15 In addition, first-principles phonon calculations with a finite displacement method have proven to be useful for accounting various structural transformation behavior of nanocrystals, such as swap motion-directed twinning of nanocrystals.57
For simulating the trajectories of materials transformation dynamics, the spatial and/or time scales of ab initio methods are prohibitively expensive. Methods with higher level of approximation and making recourse to empirical force field have shown advantages. For example, by combining analytical considerations with molecular dynamics and kinetic Monte Carlo simulations, researchers have been able to develop a comprehensive framework for their observations of nanoparticle self-assembly (Figure 3g–h). The combined in situ TEM observation and simulations allow gaining critical insight on novel crystallization pathways with nanoparticles as the building blocks.20,21 Last, innovative development in theoretical modeling enables reproducing nonequilibrium physical and chemical transformations, as observed in defect-mediated ripening of core–shell nanostructures.16
Control experiments
Carefully designed control experiments are often necessary to draw meaningful conclusions from the liquid cell TEM observations. For example, in order to discern the electron-beam effects, control experiments by systematic changing the electron-beam current density is a useful practice. For most studies, ex situ experimental results are ideal reference points for in situ liquid cell TEM studies. For instance, in the liquid cell TEM study of cobalt oxide and cobalt nickel oxide growth, it was found that after the nucleation and growth of nanoparticles into a few nanometers, the growth model transformed from 3D to 2D, surprisingly.18 A set of control experiments were conducted to exclude the possible confinement effects from a thin liquid cell, electron-beam “melting” the nanoparticles, etc. Most importantly, amazingly similar growth behavior can be found in the ex situ control experiments (Figure 3i–j).
Strategies for investigating electron-beam-sensitive chemical processes
Many chemical processes are highly sensitive to electron-beam irradiation. Strategies should be developed for liquid cell TEM study of these chemical processes. Here, we highlighted a few effective approaches that have been utilized in the study of electrochemical processes related to batteries, and electrocatalysis.
Low-dose techniques
Various advanced microscopy techniques have been developed, such as, four-dimensional scanning TEM (4D-STEM), aloof electron energy-loss spectroscopy (EELS), that can minimize electron-beam dose needed for data collection. These techniques have recently been used in liquid cell TEM studies.
In 4D-STEM experiments, a converged electron beam is scanned across the sample, and a pixelated camera records a diffraction pattern at each scan position. This 4D data set can be analyzed to produce maps of local crystal structure, crystallinity and lattice distortion. This technique has been applied to the study of beam-sensitive materials, where minimization of beam damage is achieved using the beam blanker, steering of the localized electron dose, and minimizing the fluence in the convergent beam.58,59 Four-dimensional-STEM has been introduced to study liquid samples, for example, liquid electrolytes at low temperature (−30℃) that is relevant to battery applications.60 Organic electrolytes are sensitive to electron-beam irradiation, and have weak contrast for real-space imaging. By combining the 4D-STEM technique to minimize beam damage, the reciprocal space RDF measurements for efficient use of the incident electron dose, and data analysis based on machine learning, researchers revealed the presence of short-range order (SRO) in the high–salt concentration domains of the liquid electrolyte from liquid phase separation at the low temperature (Figure 4a–b).60
Illustrations of strategies to overcome electron-beam effects in investigating electron-beam-sensitive chemical processes, including incorporating low dose techniques, ex situ situ characterization of samples from real devices, and multi-modal in operando characterization (a, b) Four-dimensional scanning transmission electron microscopy (4D-STEM) and machine learning used to reveal short-range-order in liquid electrolyte. Reprinted with permission from Reference 60. © 2023 AAAS. (a) Sample diffraction patterns of 4D-STEM data sets. Example diffraction patterns from the location marked with blue, orange, and purple boxes. (b) Visualization of the radial sum. Three phases were classified by the neural network model. Phase I (purple) represents the crystalline phases, in which the black arrows marked the corresponding Bragg peaks of LiPF6. Phase II (orange) and phase III (blue) are from the diffraction patterns with amorphous rings, which correspond to the bright and dark regions, respectively. The black arrow in phase II indicates the stronger scattered intensity at 0.23 Å−1. (c) Aloof electron energy-loss spectroscopy (EELS) study of vibrational properties of liquid water with a boron nitride liquid cell. EEL spectra of the zero loss peak of unmonochromated (black) and monochromated (red) electron probes. FWHM, full width at half maximum. Reprinted with permission from Reference 65. © 2018 Wiley. (d–g) The modifications of lithium dendritic growth with a cationic polymer (PDDA) thin film on the electrodes of an electrochemical liquid cell. Reprinted with permission from Reference 66. © 2020 Royal Society of Chemistry. (d) A schematic design of an electrochemical liquid cell. (e) A TEM image of lithium dendritic growth in an electrochemical liquid cell without the polymer film. Scale bar = 1 μm. (f) A TEM image of lithium nanogranular growth in an electrochemical liquid cell with PDDA polymer film. Scale bar = 1 μm. (g) Energy-dispersive x-ray spectroscopy (EDS) line-scan profiles of the lithium nanogranule surface showing the elemental distribution within solid-electrolyte interphase (SEI). (h) X-ray photoelectron spectroscopy (XPS) spectra of F 1s peaks without (blue) and with (red) the cationic polymer film on an ex situ sample from a coin cell experiment. (i) A schematic drawing of cross-sectional views of an ex situ sample from a coin cell. (j, k) Multimodal in operando characterization of Cu catalyst active sites in CO2 electroreduction. Reprinted with permission from Reference 67. © 2023 Springer Nature. (j) False-color dark-field 4D-STEM maps showing Cu nanograins. (k) Operando high-energy resolution fluorescence detected extended x-ray absorption fine structure (HERFD-EXAFS) of 7 nm under CO2RR conditions and upon air exposure with EXAFS after 1 h electroreduction, suggesting the presence of undercoordinated Cu sites as highlighted by the red asterisk. OCP, open circuit potential.
EELS is a vital tool for performing high spatial resolution analysis of composition, bonding and optical properties in materials. However, electron-beam damage is often the limiting factor for using EELS to characterize beam-sensitive materials or chemical processes. Aloof beam EELS with the beam positioned outside the sample, for example, tens of nanometers away from the sample, can allow materials analysis to be performed with significantly reduced electron-beam damage.61,62,63,64 Aloof EELS has been used to characterize the vibrational properties of liquid water with a boron nitride liquid cell.65 Access to the vibrational response of water requires very high energy resolution. With a momochromated beam, the energy resolution is dramatically improved (Figure 4c). The improvement coupled with the reduced background noise allows for different vibrational modes to be directly detected with the electron probe. With this technique, it is possible to capture changes in the structure and orientation of water (and other liquids) next to solid surfaces, solidification fronts, suspended nanoparticles, or in reactive systems utilizing flow and isotope labeling.3,65
Ex situ measurements with samples from real devices
Control experiments for liquid cell TEM study of highly electron-beam-sensitive chemical processes need to be carefully designed. To evaluate whether the reaction pathways are altered by electron-beam irradiation, ex situ experiments by measuring samples from real devices can be conducted. For example, a recent study reported the modifications of lithium dendritic growth with a cationic polymer film on the electrode. Using an electrochemical liquid cell with a poly(diallyldimethylammonium chloride) (PDDA) polymer film covering the electrode, the growth mode changed dramatically, from lithium dendritic growth to nanogranular growth66 (Figure 4d–f). Chemical analysis of the solid-electrolyte interphase (SEI) using EDS indicated a high concentration of LiF inner layer within the SEI (Figure 4g). In parallel, ex situ experiments, such as the measurements of Cu/carbon TEM grids with or without the polymer film placed in cycling coin cells, showed similar morphology and spectroscopic results (Figure 4h–i).
Multimodal imaging and spectroscopy
Multimodal characterization, such as using in situ TEM and in situ x-ray spectroscopy, is emerging as a powerful approach to characterize chemical processes with complex reactions scheme. It can provide valuable complimentary information. For example, in carbon dioxide electroreduction, Cu catalysts enable CO2-to-multicarbon product conversion. However, the active sites of high-performance Cu nanocatalysts are still hard to identify. A recent study reported a comprehensive investigation of the structural dynamics during the lifecycle of Cu nanocatalysts using multimodal in operando electrochemical liquid cell TEM and x-ray spectroscopy.67 It was found the nanograin boundaries of Cu metal nanoparticle aggregates, rich with undercoordinated active sites, is responsible for the C–C coupling (Figure 4j–k).
Conclusion and future outlook
Significant advances have been made in the study of various chemical processes using liquid cell TEM, including understanding and controlling of electron-beam effects. In this article, we summarized the strategies that have been developed to overcome electron-beam issues in two areas: (1) nucleation, growth, and self-assembly of nanocrystals, where the electron beam is often used to initiate the reactions; (2) highly electron-beam-sensitive chemical reactions, such as electrochemical processes. Due to the complexity of the liquid cell TEM experiments, various strategies could be considered simultaneously, such as low dose imaging, incorporating various techniques or methods, collecting more data, carefully designed control experiments, and so on. The multidisciplinary research has drawn researchers from diverse fields, including materials science, chemistry, physics, and electron microscopy to explore solutions for resolving challenging issues besides the electron-beam effects. It has opened many new opportunities by merging different expertise and approaches together. It stimulates innovations in liquid cell TEM imaging and data analysis, and fosters novel scientific discoveries.
References
H. Zheng, MRS Bull. 46(5), 443 (2021)
U. Mirsaidov, J.P. Patterson, H. Zheng, MRS Bull. 45(9), 704 (2020)
P. Ercius, J.A. Hachtel, R.F. Klie, MRS Bull. 45(9), 761 (2020)
L. Wu, J.J. Willis, I.S. McKay, B.T. Diroll, J. Qin, M. Cargnello, C.J. Tassone, Nature 548, 197 (2017)
Q. Chen, J.M. Yuk, M.R. Hauwiller, J. Park, K.S. Dae, J.S. Kim, A.P. Alivisatos, MRS Bull. 45(9), 713 (2020)
J.J. De Yoreo, N.A.J.M. Sommerdijk, Nat. Rev. Mater. 1, 16035 (2016)
W. Xu, J.S. Kong, Y.-T.E. Yeh, P. Chen, Nat. Mater. 7, 992 (2008)
P. Chen, X. Zhou, H. Shen, N.M. Andoy, E. Choudhary, K.-S. Han, G. Liu, W. Meng, Chem. Soc. Rev. 39, 4560 (2010)
M.J. Williamson, R.M. Tromp, P.M. Vereecken, R. Hull, F.M. Ross, Nat. Mater. 2, 532 (2003)
H. Zheng, R.K. Smith, Y.-W. Jun, C. Kisielowski, U. Dahmen, A.P. Alivisatos, Science 324, 1309 (2009)
H. Zheng, S.A. Claridge, A.M. Minor, A.P. Alivisatos, U. Dahmen, Nano Lett. 9, 2460 (2009)
J.M. Yuk, J. Park, P. Ercius, K. Kim, D.J. Hellebusch, M.F. Crommie, J.Y. Lee, A. Zettl, A.P. Alivisatos, Science 336, 61 (2012)
H.-G. Liao, L. Cui, S. Whitelam, H. Zheng, Science 336, 1011 (2012)
D. Li, M.H. Nielsen, J.R.I. Lee, C. Frandsen, J.F. Banfield, J.J. De Yoreo, Science 336, 1014 (2012)
H.-G. Liao, D. Zherebetskyy, H. Xin, C. Czarnik, P. Ercius, H. Elmlund, M. Pan, L.-W. Wang, H. Zheng, Science 345, 916 (2014)
Q. Zhang, X. Peng, Y. Nie, Q. Zheng, J. Shangguan, C. Zhu, K.C. Bustillo, P. Ercius, L. Wang, D.T. Limmer, H. Zheng, Nat. Commun. 13, 2211 (2022)
N.D. Loh, S. Sen, M. Bosman, S.F. Tan, J. Zhong, C.A. Nijhuis, P. Král, P. Matsudaira, U. Mirsaidov, Nat. Chem. 9, 77 (2017)
J. Yang, Z. Zeng, J. Kang, S. Betzler, C. Czarnik, X. Zhang, C. Ophus, C. Yu, K. Bustillo, M. Pan, J. Qiu, L.-W. Wang, H. Zheng, Nat. Mater. 18, 970 (2019)
B.H. Kim, J. Heo, S. Kim, C.F. Reboul, H. Chun, D. Kang, H. Bae, H. Hyun, J. Lim, H. Lee, B. Han, T. Hyeon, A.P. Alivisatos, P. Ercius, H. Elmlund, J. Park, Science 368, 60 (2020)
Z. Ou, Z. Wang, B. Luo, E. Luijten, Q. Chen, Nat. Mater. 19(4), 450 (2020)
B. Luo, Z. Wang, T. Curk, G. Watson, C. Liu, A. Kim, Z. Ou, E. Luijten, Q. Chen, Nat. Nanotechnol. 18, 589 (2023)
T.J. Woehl, T. Moser, J.E. Evans, F.M. Ross, MRS Bull. 45(9), 746 (2020)
R.F. Egerton, P. Li, M. Malac, Micron 35, 399 (2004)
X. Peng, A. Abelson, Y. Wang, C. Qian, J. Shangguan, Q. Zhang, L. Yu, Z.-W. Yin, W. Zheng, K.C. Bustillo, X. Guo, H.-G. Liao, S.-G. Sun, M. Law, H. Zheng, Chem. Mater. 31, 190 (2019)
H. Zheng, U.M. Mirsaidov, L.-W. Wang, P. Matsudaira, Nano Lett. 12, 5644 (2012)
X. Tian, U. Anand, U. Mirsaidov, H. Zheng, Small 14, 1803231 (2018)
H. Cho, M.R. Jones, S.C. Nguyen, M.R. Hauwiller, A. Zettl, A.P. Alivisatos, Nano Lett. 17, 414 (2017)
S. Keskin, N. de Jonge, Nano Lett. 18, 7435 (2018)
L.A. Bultema, R. Bücker, E.C. Schulz, F. Tellkamp, J. Gonschior, R.J.D. Miller, G.H. Kassier, Ultramicroscopy 240, 113579 (2022)
M. Wang, C. Park, T.J. Woehl, Chem. Mater. 30, 7727 (2018)
J. Korpanty, L.R. Parent, N.C. Gianneschi, Nano Lett. 21, 1141 (2021)
I.A. Moreno-Hernandez, M.F. Crook, J.C. Ondry, A.P. Alivisatos, J. Am. Chem. Soc. 143, 12082 (2021)
S. Merkens, G. De Salvo, A. Chuvilin, Nano Express 3(4), 045006 (2022)
J.M. Grogan, N.M. Schneider, F.M. Ross, H.H. Bau, Nano Lett. 14, 359 (2014)
N.M. Schneider, M.M. Norton, B.J. Mendel, J.M. Grogan, F.M. Ross, H.H. Bau, J. Phys. Chem. C 118, 22373 (2014)
B. Fritsch, T.S. Zech, M.P. Bruns, A. Körner, S. Khadivianazar, M. Wu, N. Zargar Talebi, S. Virtanen, T. Unruh, M.P.M. Jank, E. Spiecker, A. Hutzler, Adv. Sci. 9, 2202803 (2022)
J. Cookman, V. Hamilton, L.S. Price, S.R. Hall, U. Bangert, Nanoscale 12, 4636 (2020)
P. Abellan, B.L. Mehdi, L.R. Parent, M. Gu, C. Park, W. Xu, Y. Zhang, I. Arslan, J.-G. Zhang, C.-M. Wang, J.E. Evans, N.D. Browning, Nano Lett. 14, 1293 (2014)
T.J. Woehl, P. Abellan, J. Microsc. 265, 135 (2017)
Z. Cai, S. Chen, L.-W. Wang, Chem. Sci. 10, 10706 (2019)
T.J. Woehl, J.E. Evans, I. Arslan, W.D. Ristenpart, N.D. Browning, ACS Nano 6, 8599 (2012)
S. Mourdikoudis, L.M. Liz-Marzán, Chem. Mater. 25, 1465 (2013)
H.-G. Liao, H. Zheng, J. Am. Chem. Soc. 135, 5038 (2013)
J. Pal, T. Pal, Nanoscale 7, 14159 (2015)
Y. Yin, A.P. Alivisatos, Nature 437, 664 (2005)
Y. Xia, X. Xia, H.-C. Peng, J. Am. Chem. Soc. 137, 7947 (2015)
J. Ciston, I.J. Johnson, B.R. Draney, P. Ercius, E. Fong, A. Goldschmidt, J.M. Joseph, J.R. Lee, A. Mueller, C. Ophus, A. Selvarajan, D.E. Skinner, T. Stezelberger, C.S. Tindall, A.M. Minor, P. Denes, Microsc. Microanal. 25, 1930 (2019)
S. Jeon, T. Heo, S.-Y. Hwang, J. Ciston, K.C. Bustillo, B.W. Reed, J. Ham, S. Kang, S. Kim, J. Lim, K. Lim, J.S. Kim, M.-H. Kang, R.S. Bloom, S. Hong, K. Kim, A. Zettl, W.Y. Kim, P. Ercius, J. Park, W.C. Lee, Science 371, 498 (2021)
A.S. Powers, H.-G. Liao, S.N. Raja, N.D. Bronstein, A.P. Alivisatos, H. Zheng, Nano Lett. 17, 15 (2017)
L. Yao, Z. Ou, B. Luo, C. Xu, Q. Chen, ACS Cent. Sci. 6(8), 1421 (2020)
C.-C. Chen, C. Zhu, E.R. White, C.-Y. Chiu, M.C. Scott, B.C. Regan, L.D. Marks, Y. Huang, J. Miao, Nature 496, 74 (2013)
Y. Yang, C.-C. Chen, M.C. Scott, C. Ophus, R. Xu, A. Pryor, L. Wu, F. Sun, W. Theis, J. Zhou, M. Eisenbach, P.R.C. Kent, R.F. Sabirianov, H. Zeng, P. Ercius, J. Miao, Nature 542, 75 (2017)
Y. Yang, J. Zhou, F. Zhu, Y. Yuan, D.J. Chang, D.S. Kim, M. Pham, A. Rana, X. Tian, Y. Yao, S.J. Osher, A.K. Schmid, L. Hu, P. Ercius, J. Miao, Nature 592, 60 (2021)
J. Park, H. Elmlund, P. Ercius, J.M. Yuk, D.T. Limmer, Q. Chen, K. Kim, S.H. Han, D.A. Weitz, A. Zettl, A.P. Alivisatos, Science 349, 290 (2015)
J. Hong, J.-H. Bae, H. Jo, H.-Y. Park, S. Lee, S.J. Hong, H. Chun, M.K. Cho, J. Kim, J. Kim, Y. Son, H. Jin, J.-Y. Suh, S.-C. Kim, H.-K. Roh, K.H. Lee, H.-S. Kim, K.Y. Chung, C.W. Yoon, K. Lee, S.H. Kim, J.-P. Ahn, H. Baik, G.H. Kim, B. Han, S. Jin, T. Hyeon, J. Park, C.Y. Son, Y. Yang, Y.-S. Lee, S.J. Yoo, D.W. Chun, Nature 603, 631 (2022)
G. Zhu, M.L. Sushko, J.S. Loring, B.A. Legg, M. Song, J.A. Soltis, X. Huang, K.M. Rosso, J.J. De Yoreo, Nature 590, 416 (2021)
Q. Zhang, Z. Song, Y. Wang, Y. Nie, J. Wan, K.C. Bustillo, P. Ercius, L. Wang, L. Sun, H. Zheng, Sci. Adv. 8, eabp9970 (2022)
K.C. Bustillo, S.E. Zeltmann, M. Chen, J. Donohue, J. Ciston, C. Ophus, A.M. Minor, Acc. Chem. Res. 54, 2543 (2021)
W. Chen, X. Zhan, R. Yuan, S. Pidaparthy, A.X.B. Yong, H. An, Z. Tang, K. Yin, A. Patra, H. Jeong, C. Zhang, K. Ta, Z.W. Riedel, R.M. Stephens, D.P. Shoemaker, H. Yang, A.A. Gewirth, P.V. Braun, E. Ertekin, J.-M. Zuo, Q. Chen, Nat. Mater. 22, 92 (2023)
Y. Xie, J. Wang, B.H. Savitzky, Z. Chen, Y. Wang, S. Betzler, K. Bustillo, K. Persson, Y. Cui, L.-W. Wang, C. Ophus, P. Ercius, H. Zheng, Sci. Adv. 9, eadc9721 (2023)
O.L. Krivanek, T.C. Lovejoy, N. Dellby, T. Aoki, R.W. Carpenter, P. Rez, E. Soignard, J. Zhu, P.E. Batson, M.J. Lagos, R.F. Egerton, P.A. Crozier, Nature 514, 209 (2014)
P. Rez, T. Aoki, K. March, D. Gur, O.L. Krivanek, N. Dellby, T.C. Lovejoy, S.G. Wolf, H. Cohen, Nat. Commun. 7, 10945 (2016)
P.A. Crozier, Ultramicroscopy 180, 104 (2017)
M.J. Lagos, A. Trügler, U. Hohenester, P.E. Batson, Nature 543, 529 (2017)
J.R. Jokisaari, J.A. Hachtel, X. Hu, A. Mukherjee, C. Wang, A. Konecna, T.C. Lovejoy, N. Dellby, J. Aizpurua, O.L. Krivanek, J.-C. Idrobo, R.F. Klie, Adv. Mater. 30, 1802702 (2018)
S.-Y. Lee, J. Shangguan, J. Alvarado, S. Betzler, S.J. Harris, M.M. Doeff, H. Zheng, Energy Environ. Sci. 13(6), 1832 (2020)
Y. Yang, S. Louisia, S. Yu, J. Jin, I. Roh, C. Chen, M.V. Fonseca Guzman, J. Feijóo, P.-C. Chen, H. Wang, C.J. Pollock, X. Huang, Y.-T. Shao, C. Wang, D.A. Muller, H.D. Abruña, P. Yang, Nature 614(7947), 262 (2023)
S.J. Pennycook, M. Varela, C.J.D. Hetherington, A.I. Kirkland, MRS Bull. 31(1), 36 (2006)
I.M. Abrams, J.W. McBain, J. Appl. Phys. 15, 607 (1944)
Funding
This work was supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES), Materials Science and Engineering Division under Contract No. DE-AC02-05-CH11231 within the KC22ZH program. Work at the Molecular Foundry is supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. W.Z. acknowledges the funding support of the Fundamental Research Program of Shanxi Province (Grant No. 202103021223183). She also thanks J. Wu for his support while she visited LBNL and UC Berkeley. D.L. acknowledges the Kwanjeong Study Abroad Scholarship from the KEF (Kwanjeong Educational Foundation) (KEF-2019).
Author information
Authors and Affiliations
Contributions
H.Z. conceived and supervised this work. W.Z., D.L., and H.Z. made figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, W., Lee, D. & Zheng, H. Strategies to overcome electron-beam issues in liquid phase TEM: Study of chemical processes. MRS Bulletin 49, 205–213 (2024). https://doi.org/10.1557/s43577-024-00661-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-024-00661-5
Taxonomy
- Chemical reaction
- Electron irradiation
- In situ
- Liquid
- Nanoscale
- Radiation effects
- Scanning transmission electron microscopy (STEM)
- Transmission electron microscopy (TEM)
- In situ transmission electron microscopy
- Liquid phase electron microscopy
- Liquid phase TEM
- Liquid cell
- Electron-beam effects
- Electron-beam-sensitive materials
- Electron-beam related chemical processes