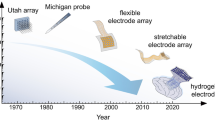
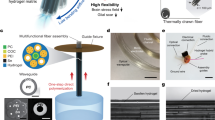
Abstract
Hydrogels are a class of soft materials, which display unique biomimetic properties to biological tissues. Their mechanical properties, high water content, and porosity resemble that of extracellular matrix so that cell growth and proliferation can be reliably supported. In vitro studies report that mechanosensitive cells found in the central nervous system, such as astrocytes and glia, display reduced activation, thus promoting lower foreign body reaction, when cultured on hydrogel substrates of <1-kPa modulus. This observation provides an opportunity to explore whether soft hydrogels should be integrated in or form implantable neural interfaces and offer long-term biointegrated neurotechnologies. This article highlights recent progress in hydrogel materials and associated technologies for the design of implantable bioelectronics. Essential structural, mechanical, and electronical properties of hydrogels and composite hydrogels are briefly reviewed. Manufacturing methods suitable for these multiscale and multifunctional materials are presented. The final section presents hydrogel-based implantable bioelectronics for the brain and outlines current challenges and future opportunities.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1960, William Chardack and colleagues implanted the first cardiac pacemaker into a 77-year-old man who lived for 10 months after the surgery;1 nine other patients received the device that year, several of whom lived more than 20 years after the procedure. Today, pacemakers are routinely implanted worldwide, with more than 3 million people benefiting from the electrical modulation device. This first clinically successful device launched the domain of implantable medical devices and called for innovation in implantable materials and electronics, system integration, miniaturization, and biointegration. Implantable neural interfaces such as deep brain or spinal cord stimulators and cochlear implants follow this technological trend.2,3,4 While many efforts are ongoing in the design and optimization of the electronic components (i.e., artificial intelligence [AI]-based chips), batteries, and radiofrequency technology, innovation in materials for packaging and transducing functions is essential to engineer the next generation of therapeutical bioelectronic implants.5 A recurrent challenge is biointegration, which supports the long-term stability and function of the artificial device with the body. Foreign body reaction (FBR)6 orchestrated by the immune system targets the artificial implant over time through mechanisms such as acute secretion of aggressive chemicals like reactive oxygen species (ROS) and chronic fibrotic encapsulation through multiple compact layers of fused macrophages. FBR hinders the chronic performance of bioelectronic implants and is particularly challenging for miniaturized and recording bioelectronic implants.6,7,8 The mechanical and chemical mismatch between implant materials and the target tissue is identified as a prime trigger behind the formation and evolution of the FBR following implantation. In brief, FBR is initiated immediately following implantation, when tissue laceration and bleeding trigger the secretion of chemokine and pro-inflammatory proteins that adhere to the surface of the implant and direct the action of immune cells nearby. Chronically, immune cells such as macrophages surround the implant and create a fibrotic capsule through the simultaneous aggregation, fusion, and secretion of collagen. Mechanical mismatch between usually stiff and static implant materials (that display elastic moduli from tens of MPa to hundreds of GPa) and soft and dynamic tissue (100 Pa to 1 MPa in elastic modulus) maintains and even worsens FBR because of continuous displacement and stress at the implant–tissue interface.9
The implementation of hydrogels, three-dimensional (3D) polymer networks retaining an elevated water content, within implantable devices has gained traction as a design strategy to control and/or mitigate FBR.8 Hydrogels display interesting properties to support biointegration; they are water-based hydrophilic polymeric materials similar to living tissues; their mechanical properties could be tailored to mimic those of the target tissue; they can be engineered to provide spatial and temporal release of therapeutical agents, including drugs, cell, or anti-inflammatory molecules.10 Moreover, multiple approaches also enable the engineering of electrically conductive hydrogels, promoting charge transfer at the interface of the bioelectronic device and target tissue and minimizing FBR.11 To date, hydrogels are mostly integrated in implants as surface coatings, but recent advances explore their use as core implant materials.
Here, we provide an overview on how hydrogels are formulated, synthesized, and integrated with bioelectronic implants and then focus on electrically conductive formulations, including composites. We report on their tunable and multifunctional properties and highlight how they could improve performance in state-of-the-art implantable bioelectronic devices.
Building blocks and properties of hydrogels
Hydrogels are composed of a cross-linked polymer network and a large amount of water. Both natural and synthetic hydrogels have been used for biomedical applications, the most common ones include collagen, alginate, gelatin, fibrin, hyaluronic acid (HA), poly(ethylene glycol) (PEG), polyacrylamide (PAAm), poly(vinyl alcohol) (PVA), and poly(hydroxyethyl methacrylate) (PHEMA). The 3D polymer network is formed through chemical or physical cross-links or chain entanglement (Figure 1a) and does not typically dissolve in water. Gelation is the essential process producing the hydrogel, characterized by either chemical interactions of monomers covalently assembled in polymer chains and condensed through cross-linkers, initiators, or reactive compounds or physical interlocking of the polymer chains via ionic gelation, electrostatic interactions, or hydrogen bonding through molecular entanglements and hydrophobic associations.12,13 Biodegradable hydrogels can also be designed by incorporation of labile chemical bonds, which are susceptible to hydrolytic or enzymatic cleavage.14 Hydrogels are therefore multiscale materials, with millimeter to sub-nanometer features. Bulk volumes will determine fabrication methods of hydrogel-based devices. Microstructures (i.e., mesh size and porosity of the hydrogel) control the swelling rate, chemical diffusion within, and mechanical properties of the gel. Nanometric features driven by chemical or electrostatic coupling of cross-linkers and polymer chains and eventual chemical functionalization govern local interactions.
Hydrogels: (a) Schematic illustration of the main building blocks forming hydrogels and conductive hydrogels. (b) Quantification of the elastic modulus, electrical conductivity, and viscoelasticity (tan(δ)) of various tissues and conductive composites. CNT, carbon nanotube. Adapted from Reference 15.
Hydrogels are cross-linked hydrophilic polymers, which, upon addition of water, can swell—dramatically increasing their volume depending on their preparation.16,17 This 3D architecture greatly resembles the natural structure of the extracellular matrix (ECM) in terms of porosity, water content, and mechanical properties.
The mechanical properties of hydrogels are particularly appealing for integration in implantable biomimetic devices. Tuning of cross-linking ratio and molecular weight of the polymeric chains allow the design of hydrogels with soft tissue-like properties, such as softness in the 1 Pa to 1 MPa range and high stretchability (~20–75% reversible elongation)13 as well as controlled swelling and degradability features.18 Hydrogels are mostly viscoelastic materials and thus display stress relaxation in response to deformation.12,13,18,19 Covalently cross-linked hydrogels show more viscoelastic behavior than physically cross-linked hydrogels with a stress relaxation time in the range of ∼10–200 s. Therefore, hydrogels cannot only mimic the basic mechanical properties of the tissue, but also reduce shear strains at the tissue interface, those occurring due to micromotion and tethering.20
To date, hydrogels are mostly implemented as a coating material of a variety of bioelectronic implant surfaces. Due to the high water content and limited structural stability, using hydrogel as an implant substrate or carrier imposes many challenges in terms of electrical insulation, handling, delivery to the target site, and long-term mechanical stability.21
Another implementation of hydrogels in implantable devices is their use as electrical or electronic/ionic interface material. To date, engineering hydrogels with high electrical conductivity without compromising their mechanical compliance and processability remains a significant challenge.22 Electrical interconnects (i.e., conductive tracks with low resistance) are rarely prepared from conductive polymers (CPs), let alone conductive hydrogels (CHs). However, the latter can be implemented as conductive electrode coatings, ensuring locally both efficient charge transfer and mechanical compliance. Considering morphology and material choices, a myriad of approaches have been used to employ different combinations of conductive composite fillers, polymers with amorphous and crystalline regions, and hydrogel host to form conductive hydrogels (Figure 1a).23 It is generally considered that the hydrophilic hydrogel matrix creates a 3D surface of conductive material, which is then available for charge transfer.
Blending with conductive components, such as conductive polymers, leverages the CP electrical properties while addressing the mechanical limitations (stiffness) of the dry CP conductors. Process routes differ in the polymerization order of the two components (hydrogel and CP), depending on each specific chemistry. The CP could be introduced into the matrix either by polymerization inside a prefabricated hydrogel or in parallel with the hydrogel cross-linking reaction. Alternatively, pre-polymerized CP could be blended with the hydrogel precursors and integrated within the hydrogel matrix following its cross-linking. Electronic conduction is achieved by coupling π bonds of the CP (polythiophene (PT), polypyrrole (PPy), PANI, PEDOT). Alternatively, the hydrogel could be blended with percolating conducting fillers (e.g., nanoparticles, flakes, or nanotubes (carbon nanotubes [CNTs]), metal nanoparticles, and graphene). Typical conductivity of such conductive hydrogels is in the 10–4–102 S/m range (Figure 1b).24 Electrochemical properties, such as impedance, charge-storage, and charge-injection capacitances (CSC and CIC, respectively), of metallic microelectrodes coated with conductive hydrogel are significantly improved compared to that of plain metal or CP-coated microelectrodes.25 Mechanically tailoring conductive hydrogels with the aforementioned ways could lead to different mechanical characteristics, for example, toughness, elasticity, and strength may not be sustained with the percolation conductive fillers to hydrogel matrices. Even if one can achieve high electrical conductivity with fillers, their inhomogeneous distribution could cause weak adhesion and undesirable mesh size. On the other hand, intrinsically ionic and electronic hydrogels can enable high stretchability (>100% strain) and tissue-like softness (<100 kPa). Hence, the development of hydrogel networks by incorporating multifunctional materials is a notable challenge that one should always consider to achieve desired hydrogel functionalities.26
Finally, free ions can diffuse through the conducting hydrogel matrix, which then becomes a 3D interface promoting ionic-electronic charge transfer. Ions from the extracellular medium such as those induced by neuron firing activity could then penetrate the volume of the conducting hydrogel and modulate its electrical local conductance. Conductive hydrogels can thus offer mixed ionic/electronic charge transport.
Processing and fabrication of hydrogels
Several processes can be distinguished for the preparation, synthesis, and fabrication of hydrogels and hydrogel composites for implantable bioelectronics. The type of process is related to the final application, the targeted softness, the materials used, and the desired resolution. Table I summarizes the main processes for the fabrication of hydrogels, with indication of the hydrogel structures, resolution, and technical properties. Hydrogels are generally synthesized by cross-linking of polymers until they reach the gelation point. Functional hydrogels for bioelectronics are prepared using the following main methods: (1) by self-polymerization or self-assembly of conductive polymers/fillers that produce single-component soft networks, (2) by doping conductive polymers/fillers to produce interpenetrating hydrogel networks, and (3) by diffusing/embedding free ions or conductive fillers into nonconductive hydrogel networks.26 Using high contents of conductive fillers in a hydrogel matrix is useful to enhance the conductivity owing to a stronger connectivity and percolation, but on the other hand it could hinder high compliance, which is required for implantable bioelectronics. In order to increase the stretchability, different types of fillers can be used, such as PEDOT:PSS nanofibrils,27 graphene oxide flakes,28 carbon nanotubes (CNTs),29 ionic liquid plasticizers,30 or macromolecular/microspheres as energy-dissipating centers.31 As prime technique, the interactions between these fillers and the hydrogel networks can be engineered to build noncovalent bonds with polymer chains, but other strategies can be adopted to achieve toughness and stretchability (e.g., using molecular sliding mechanisms, prestretching/folding templates or wavy, and serpentines-based structures).32,33,34,35,36 Besides compliance, self-healing properties are extremely desirable for hydrogels used in bioelectronic implants; the degradation of the gels due to mechanical deformations and stresses, in fact, contribute to decrease their service time and impede long-term operations. Self-healing hydrogels can be obtained by designing reversible weak interactions in the polymer networks (e.g., electrostatic,37 hydrophobic,38 hydrogen bond,39 Diels–Alder reaction,40 imine bond,41 coordination bond,42 and reversible radical reaction43), which are reformed under the application of an external stress and restore the mechanical and functional properties of the hydrogel.26
Micromolding is the most common technique for preparing and patterning hydrogels for neural implantable probes (Figure 2). Tringides et al.,24 for instance, engineered a surface microelectrode array using hydrogels as the outer layers, in particular, a hydrogel-based conductor made from an ionically conductive alginate matrix enhanced with carbon nanomaterials. The authors cast nanoporous/microporous conductive gels in molds, resulting in tracks that could conform to the complicated geometries of the brain sulci and vasculature; therefore, the viscoelastic surface electrode arrays offered promising bioengineering applications for recordings and stimulation. Spencer et al.20 also studied the ability of soft poly(ethylene glycol) (PEG) hydrogel coatings to modulate glial scar formation around neural implants; the coatings significantly reduced the local strain resulting from micromotion around the implants, thus reducing scarring in vivo.
Schematic illustration of the main techniques for the fabrication and preparation of hydrogels for implantable bioelectronics, classified in terms of resolution and obtainable hydrogel structures (2D, 2D+, 3D macro, 3D micro/nano). Techniques highlighted in red have been employed in the preparation of neural implants. Reproduced with permission from the following references: Micromolding,20 melt electrospinning writing,45 screen printing,46 fused deposition modeling,47 stereolithography,48 thermal drawing,49 3D bio-plotting,50 two-photon lithography,51 UV photolithography,52 inkjet printing,53 e-beam lithography,54 self-assembly,101 laser-induced forward transfer,56 and electrochemical gelation.57
Three-dimensional printing is a promising technique for the preparation of hydrogels for implantable bioelectronics. Biomaterial inks can be used to feed 3D printing machines and produce 3D customized porous architectures and scaffolds. Against simple casting in preformed molds, additive manufacturing is advantageous for the fabrication of hydrogels and highly hydrated 3D polymer networks, because it provides accuracy, customization, a wide variety of suitable materials, and high reproducibility.58 The choice of material inks is crucial especially for 3D bioprinting of cell-laden hydrogels and for 4D printing of stimuli-responsive hydrogels:59,60 in fact, the hydrogel-forming polymers suitable for 3D printing require an accurate control of their material properties, degradability, biocompatibility, mechanical integrity, gelation mechanism, and functional properties. Li et al.61 provided a comprehensive review of the 3D printing techniques for hydrogels as well as guidelines for a rational design of printable hydrogel-based ink materials for 3D/4D printing/bioprinting. Three main techniques are described: extrusion printing, inkjet printing, and laser-based printing;61 although only few examples can be found in the literature of hydrogels prepared through these techniques for neuroelectronic applications, it is worthy to highlight them as useful and promising tools. Extrusion printing is the most common technique to produce macroscopic objects and structures via the layer-by-layer additive deposition of molten polymers or polymeric solutions passing through an extrusion print head. There are two main extrusion printing techniques: melting-based processes and dissolution-based processes,37 depending if the precursor polymer fed into the extrusion tool is molten or dissolved in a solvent to achieve an extrudable solution. Examples of melting-based processes are the fused deposition modeling47,63 and melt electrospinning writing.45 Three-dimensional plotting or direct ink writing (DIW) is instead an example of the dissolution-based technique; it is based on a nozzle and a cartridge that can move horizontally and vertically and that provides a 3D dispensing of the polymer solution in a laminar fashion and controlled by tunable air pressure.50 Wang et al.,48 for instance, proposed electrically conductive 3D periodic hydrogel-based micro-scaffolds fabricated through a particle-free DIW approach for use as neuronal growth and for extracellular electrophysiological recording of neuronal activities.
Inkjet printing is a noncontact 3D printing technique consisting of reproducing a digital pattern onto a substrate using droplets of proper material inks. The ink can be administered continuously (continuous ejection system) in the form of a jet or in a pulsed way in order to create discontinuous droplets with predefined volume (drop-on-demand jetting system).64,65 Inkjet printing represents a promising technique with potential applications owing to a high spatial resolution and the possibility to deposit multiple materials integrated into one structure; on the other hand, the printed ink must have very low viscosity to create microdroplets and generally post-deposition treatments are used to form the final structures.66 As reported by Negro et al.53 there are two critical requirements for successfully performing inkjet printing of high-resolution 3D hydrogel structures: (1) the dispensed microdroplets must retain a 3D structure and (2) they must undergo a very rapid gelation step. The same authors demonstrated an optimized inkjet printing process to structure alginate into a tissue-like branched microvasculature suitable for physiologically relevant flow.67 Adly et al.68 used a carbon nanoparticle ink to print a hydrogel multielectrode array for extracellular recording of the action potential from HL-1 cells.
Through the porous bulk structure of 3D hydrogel matrices, chemical and physical interactions can be displayed, therefore ECM-like environment mimicking. Screen printing is a low-cost alternative to other 3D (bio)printing techniques. Alternatively, it has been used to print 2D (planar) and 2D+ (stacked planar) nano/microscale layers/films/flakes/dots of conductive and dielectric materials onto printed circuit boards for applications in electronics, sensing, and microfluidics. However, it can also be adopted to produce 3D structures of gels by successive screen printing of sliced layers.69 Pandala et al.70 developed a 3D screen printing process for the creation of 3D structures of hydrogels containing live cells. Stencil masks used for screen printing can be made of inexpensive materials and sterilized to go in contact with biomaterials and patterns of cells. Additionally, different viscosities can be used for the screen-printed inks, and several substrates can be adopted (i.e., absorbent materials [paper, cloth, etc.] or nonabsorbent surfaces [glass, metal, ceramics, plastics]), making the technique more adaptable and versatile than others. Shur et al.46 reported on the preparation and patterning of a soft electrode coating of neural interfacing devices based on a screen-printable conducting hydrogel of polyacrylamide and poly(3,4-ethylenedioxythiophene) poly(styrene sulfonate). The coating formulation exhibited optimal adhesion with elastomeric substrates for soft neural interfaces and it was integrated within a 4 × 4 microelectrode array for electrocorticography.
In the context of the creation of 3D hydrogel networks, thermal drawing is another valuable choice. This technique has been used recently for producing multifunctional, fiber-based neural probes and interfaces that can interrogate neuronal circuits with electrical, optical, and chemical functionalities.11,49,71,72 These probes have been demonstrated to enable one-step optogenetics, in vivo photo-pharmacology and in situ electrochemical synthesis of molecules for neuromodulation. The process consists of fabricating a macroscopic model called preform and drawing it into fibers at the kilometer-length scale and with microscale features. Tabet et al.49 reported on a rapid, robust, and modular method based on solvent evaporation or entrapment-driven integration process for the creation of multimodalities fiber-based neural interfaces all encased within a copolymer of water-soluble PEG tethered to water-insoluble poly(urethane) (PU-PEG). The resulting cladding forms hydrogel upon exposure to water, enabling the controlled release of molecules and nanomaterials in vitro and in vivo. Park et al.11 proposed hydrogel hybrid probes, by first fabricating individual functional fibers via thermal drawing, based on polycarbonate core and cyclic olefin copolymer cladding, Tin microelectrode encapsulated within polyetherimide insulating cladding, and then hybridizing them with a hydrogel matrix, thus leveraging hydrogel’s favorable mechanical and chemical properties to reduce the impact on the surrounding tissue.73 Thermal drawing, however, is limited to some material requirements; in fact, the components of a fiber-based probe must have similar glass transition temperature (for polymers) or melting temperatures (for metals), and similar viscosities to guarantee a stable cross section during drawing.
Three-dimensional printing assisted with light consists of inducing the polymerization of a liquid photocurable resin through a UV laser source. The most common method is the stereolithography, in which a system allows for the X and Y movements of the laser beam and for the Z movement of the fabrication platform. The latter moves layer by layer after curing each layer of resin to finally create a solidified 3D structure, which usually undergoes post-treatments of washing-off the residual resin. The resolution of this technique is limited to 30 µm and thus it is generally used for macroscopic constructs and for patterned 3D or 2D + layers. Wang et al.74 presented a conductive nanocomposite network hydrogel fabricated by projection microstereolithography-based 3D printing; the hydrogels can be used as flexible electrodes for capturing human electrophysiological signals (EOG and EEG).
The laser-induced forward transfer technique is an alternative to the stereolithography and more suitable for high-resolution patterns or for 3D bioprinting of cells/hydrogels. It is a noncontact, nozzle-free method that consists of a pulsed laser focused onto a thin layer of laser-absorbing materials (hydrogel), a donor laser-transparent substrate (quartz or glass), and an acceptor substrate where the ink is transferred.56,75,76 When the energy carried out by the laser passes over a specific threshold, the ink material is ejected from the donor and propelled to the acceptor. Another high-resolution technique that can be used to construct nanometric hydrogel structures and patterns is the two-photon polymerization (2PP) or two-photon lithography (2PL). It consists of a near-infrared femtosecond laser focused into the targeted volume of a photosensitive material. Using a proper photo-initiator resin, the material can absorb two photons of 800-nm wavelength in place of one UV photon of 400-nm wavelength, triggering the polymerization only in the focal point and creating a solid voxel with resolution lower than the diffraction limit of the applied light.51,77,78
Other methods for printing hydrogels for implantable bioelectronics include the electrochemical gelation (or electro-gelation, electrodeposition), which consists of the deposition of hydrogel onto an electrode surface, by changing the solubility of the surrounding gelatin component through the reduction or oxidation of a chemical species.57 Differently from gels formed from the bulk phase, hydrogels fabricated via electro-gelation can be deposited on any conductive surface and they can be used to embed nanomaterials, enzymes, drugs or live cells, with applications in regenerative medicine, biosensors, and implantable bioelectronics.79,80,81 For instance, Wang et al.82 performed electrodeposition of alginate with PEDOT:PSS-coated multiwalled carbon nanotubes onto a microwire neural electrode; the conductive hydrogel soft interpenetrating network improved the neural interface with higher charge-storage capacity and lower electrochemical impedance.
Implementation of hydrogels in implantable neural interfaces
Hydrogels contribute to improve the functionality of implantable bioelectronic devices in a wide range of applications83 (electrode systems,84 tissue repair,85 drug delivery,86 or responsive bioactuators87) (Figure 3).
Examples of multifunctional hydrogels leveraging biological, mechanical, and electrical properties for implantable neuroelectronic interfaces. (a) Conductive hydrogel for electrode coating.89 (b) Soft electrode array entirely prepared with viscoelastic materials.88 (c) Adaptive, multifunctional hydrogel-based probe for the brain.11
Hydrogels can improve the biocompatibility of implantable devices by providing immediate antibleeding,90 acute antibacterial,91 and chronic antifouling effects.92 Hemostatic function can be achieved using hydrogel coatings based on mussel-inspired catechol-chitosan chemistry, which instantly generates a coagulant adhesive layer when in contact with blood plasma proteins.93,94 Bacteriostatic function can be achieved by incorporating antibiotic agents in the hydrogel that will then be released to the tissue or using intrinsically antibacterial materials, such as hyaluronic acid (HA) or poly(ethylene glycol) (PEG).95,96
To prevent the progressive formation of a fibrotic scar-tissue capsule around implantable devices, the latter can be coated by antifouling hydrogels with reduced adhesion and activation of pro-inflammatory cells, such as myofibroblasts and macrophages. This nonspecific protein adsorption resistance can be achieved with hydrogels based on zwitterionic groups,15,97,98 thanks to its surface hydration layer or bacterial cellulose99 and its strong hydrophilicity.
As electrode coating, conductive hydrogels offer superior charge transfer and mechanical compliance (Figure 3a). One of the key requirements for this implementation is maintaining a stable interface with the tissue. Reliable adhesion of the hydrogel coating to the underlying electrodes needs to be ensured over time. Surface treatment that introduces covalent anchoring to the receiving electrodes is essential.46
Figure 3b displays a surface microelectrode array that is made entirely of viscoelastic hydrogels to match both the stiffness and relaxation behavior of soft biological tissue. Conductive tracks are patterned with a matrix of ionically conductive alginate that carries carbon-based fillers. The tough yet viscoelastic encapsulation bilayer flows to conform the convoluted surface of the cortex and ensures reliable electrical insulation. This combination of conducting and insulating hydrogels is produced with top-down manufacturing and offers compatibility with standard electrophysiology platforms.
Swelling cycles of hydrogels, running dry stiff to hydrated soft gels, offer a design opportunity for miniaturized flexible and implantable neural interfaces. Their insertion into the brain is challenged by their corresponding low buckling force. The high elastic modulus of dry hydrogel films used as transient insertion carrier or implant coating enables its insertion; once implanted, the hydrogel hydrates and swells to either degrade or soften further matching the mechanics of the surrounding neural tissue.55,100 A recent example of this strategy is presented in Figure 3c. Functional thermally drawn fibers are integrated into a soft hydrogel matrix (PAAm-Alg hydrogel) that offers brain-like softness, mechanical robustness, chemical stability over time in vivo, and a reliable bonding with the polymers constituting the functional fibers.11 These hybrid brain probes possess adaptive bending stiffness determined by the hydration states of the hydrogel matrix. This enables their direct insertion into the deep brain regions, while minimizing tissue damage associated with the brain micromotion after implantation. This compelling demonstration is an example of innovative materials engineering to tailor the mechanical properties of the neural interface without compromising on its function.101
Conclusion
Implantable devices alleviate consequences of traumatic or disease-related conditions to a few million people worldwide. Although a handful of designs and associated technologies and materials are dominating the current clinical device portfolio, the last decade is propelling materials’ innovation and manufacturing strategies toward the next generation of therapeutical implants that should be biomimetic, multifunctional, and lifelong standing. Hydrogels are already used in many clinical applications, mostly for scaffolding and drug delivery. Recent progress now aims at integrating these soft materials into bioelectronic implants and leveraging their customized structure, chemistry, and physical properties. Stealth implants that display tissue-like physical properties and miniaturized geometry promise minimal disruption of the glial and neural network and reliable and stable tissue–implant communication. In addition to mechanical biointegration, hydrogels in their conductive form also emerge as a promising class of transducing materials for bioelectronics; they offer a unique ionic-electronic transport and transfer network for recording and stimulation devices. Efforts in dispensing and patterning these soft conducting gels are now needed to reliably integrate them in neural implants with high spatial electrode distribution. Implant prototypes made entirely from hydrogels become possible; solutions to reliably pattern and electrically insulate conducting gel tracks without compromising the form factor of the implant have yet to be developed. Hydrogels offer a broad range of options in materials’ design, structure, and properties that will translate in diverse implementation in implantable bioelectronics.
References
W.M. Chardack, A.A. Gage, W. Greatbatch, Surgery 48, 643 (1960)
T. Hyakumura, U. Aregueta-Robles, W. Duan, J. Villalobos, W.K. Adams, L. Poole-Warren, J.B. Fallon, Front. Neurosci. 15 (2021). https://doi.org/10.3389/fnins.2021.761525
L.H. Nguyen, M. Gao, J. Lin, W. Wu, J. Wang, S.Y. Chew, Sci. Rep. 7, 42212 (2017)
A.A. Guex, N. Vachicouras, A.E. Hight, M.C. Brown, D.J. Lee, S.P. Lacour, J. Mater. Chem. B. 3, 5021 (2015)
S. Choi, H. Lee, R. Ghaffari, T. Hyeon, D.H. Kim, Adv. Mater. 28, 4203 (2016)
J.M. Anderson, A. Rodriguez, D.T. Chang, Semin. Immunol. 20, 86 (2008)
D.G. Barone, A. Carnicer-Lombarte, P. Tourlomousis, R.S. Hamilton, M. Prater, A.L. Rutz, I.B. Dimov, G.G. Malliaras, S.P. Lacour, A.A.B. Robertson, K. Franze, J.W. Fawcett, C.E. Bryant, Proc. Natl. Acad. Sci. U.S.A. 119, e2115857119 (2022)
A. Chen, D. Chen, K. Lv, G. Li, J. Pan, D. Ma, J. Tang, H. Zhang, Adv. Healthc. Mater. 12, 2200807 (2023)
X. Tang, H. Shen, S. Zhao, N. Li, J. Liu, Nat Electron. 6, 109 (2023)
J. Li, D.J. Mooney, Nat. Rev. Mater. 1(12), 16071 (2016)
S. Park, H. Yuk, R. Zhao, Y.S. Yim, E.W. Woldeghebriel, J. Kang, A. Canales, Y. Fink, G.B. Choi, X. Zhao, P. Anikeeva, Nat. Commun. 12, 3435 (2021)
J.A. Burdick, M.M. Stevens, “Biomedical Hydrogels,” in Biomaterials, Artificial Organs and Tissue Engineering, ed. by L.L. Hench, J.R. Jones (Elsevier, 2005), chap. 11, p. 107
Y.S. Zhang, A. Khademhosseini, Science 356, 21 (2017)
N.A. Peppas, J.Z. Hilt, A. Khademhosseini, R. Langer, Adv. Mater. 18, 1345 (2006)
X. Wang, J. Wang, Y. Yu, L. Yu, Y. Wang, K. Ren, J. Ji, Compos. B Eng. 244, 110164 (2022)
E. Jabbari, S. Nozari, Eur. Polym. J. 36, 2685 (2000)
K. Hoshino, T. Nakajima, T. Matsuda, T. Sakai, J.P. Gong, Soft Matter 14, 9693 (2018)
H.J. Kong, D. Kaigler, K. Kim, D.J. Mooney, Biomacromolecules 5(5), 1720 (2004)
O. Chaudhuri, Biomater. Sci. 5, 1480 (2017)
K.C. Spencer, J.C. Sy, K.B. Ramadi, A.M. Graybiel, R. Langer, M.J. Cima, Sci. Rep. 7, 1952 (2017)
J. Goding, C. Vallejo-Giraldo, O. Syed, R. Green, J. Mater. Chem. B 7, 1625 (2019)
H. Yuk, B. Lu, X. Zhao, Chem. Soc. Rev. 48, 1642 (2019)
T. Rafeedi, D.J. Lipomi, Science 378, 1174 (2022)
C.M. Tringides, N. Vachicouras, I. de Lázaro, H. Wang, A. Trouillet, B.R. Seo, A. Elosegui-Artola, F. Fallegger, Y. Shin, C. Casiraghi, K. Kostarelos, S.P. Lacour, D.J. Mooney, Nat. Nanotechnol. 16(9), 1019 (2021)
D.-H. Kim, M. Abidian, D.C. Martin, J. Biomed. Mater. Res. 71A, 577 (2004)
F. Fu, J. Wang, H. Zeng, J. Yu, ACS Mater. Lett. 2, 1287 (2020)
B. Lu, H. Yuk, S. Lin, N. Jian, K. Qu, J. Xu, X. Zhao, Nat. Commun. 10, 1043 (2019)
D. Gan, Z. Huang, X. Wang, L. Jiang, C. Wang, M. Zhu, F. Ren, L. Fang, K. Wang, C. Xie, X. Lu, Adv. Funct. Mater. 30, 1907678 (2020)
G. Cai, J. Wang, K. Qian, J. Chen, S. Li, P.S. Lee, Adv. Sci. 4, 1600190 (2017)
Y. Wang, C. Zhu, R. Pfattner, H. Yan, L. Jin, S. Chen, F. Molina-Lopez, F. Lissel, J. Liu, N.I. Rabiah, Z. Chen, J.W. Chung, C. Linder, M.F. Toney, B. Murmann, Z. Bao, Sci. Adv. 3, e1602076 (2017)
J. Duan, X. Liang, J. Guo, K. Zhu, L. Zhang, Adv. Mater. 28, 8037 (2016)
C. Xu, B. Ma, S. Yuan, C. Zhao, H. Liu, Adv. Electron. Mater. 6, 1900721 (2020)
S. Huang, Y. Liu, Y. Zhao, Z. Ren, C.F. Guo, Adv. Funct. Mater. 29, 1805924 (2019)
S. Choi, S.I. Han, D. Kim, T. Hyeon, D.H. Kim, Chem. Soc. Rev. 48, 1566 (2019)
A. Bin Imran, K. Esaki, H. Gotoh, T. Seki, K. Ito, Y. Sakai, Y. Takeoka, Nat. Commun. 5, 5124 (2014)
Y. Zhao, W. Yang, Y.J. Tan, S. Li, X. Zeng, Z. Liu, B.C.K. Tee, APL Mater. 7, 031508 (2019)
D. Wan, Q. Jiang, Y. Song, J. Pan, T. Qi, G.L. Li, ACS Appl. Polym. Mater. 2, 879 (2020)
Y. Deng, I. Hussain, M. Kang, K. Li, F. Yao, S. Liu, G. Fu, Chem. Eng. J. 353, 900 (2018)
S. Liu, O. Oderinde, I. Hussain, F. Yao, G. Fu, Polymer 144, 111 (2018)
F. Yu, X. Cao, J. Du, G. Wang, X. Chen, ACS Appl. Mater. Interfaces 7, 24023 (2015)
M. Wu, J. Chen, W. Huang, B. Yan, Q. Peng, J. Liu, L. Chen, H. Zeng, Biomacromolecules 21, 2409 (2020)
Y. Shi, M. Wang, C. Ma, Y. Wang, X. Li, G. Yu, Nano Lett. 15, 6276 (2015)
M.M. Perera, N. Ayres, Polym. Chem. 11, 1410 (2020)
J. Yeh, Y. Ling, J.M. Karp, J. Gantz, A. Chandawarkar, G. Eng, J. Blumling III, R. Langer, A. Khademhosseini, Biomaterials 27, 5391 (2006)
Y. Jin, Q. Gao, C. Xie, G. Li, J. Du, J. Fu, Y. He, Mater. Des. 185, 108274 (2020)
M. Shur, F. Fallegger, E. Pirondini, A. Roux, A. Bichat, Q. Barraud, G. Courtine, S.P. Lacour, ACS Appl. Bio Mater. 3(7), 4388 (2020)
H. Nassar, R. Dahiya, Adv. Intell. Syst. 3, 2100102 (2021)
C. Wang, S.S. Rubakhin, M.J. Enright, J.V. Sweedler, R.G. Nuzzo, Adv. Funct. Mater. 31, 2010246 (2021)
A. Tabet, M.J. Antonini, A. Sahasrabudhe, J. Park, D. Rosenfeld, F. Koehler, H. Yuk, S. Hanson, J. Stinson, M. Stok, X. Zhao, C. Wang, P. Anikeeva, ACS Cent. Sci. 7, 1516 (2021)
T. Pan, W. Song, X. Cao, Y. Wang, J. Mater. Sci. Technol. 32, 889 (2016)
O. Kufelt, A. El-Tamer, C. Sehring, M. Meißner, S. Schlie-Wolter, B.N. Chichkov, Acta Biomater. 18, 186 (2015)
A. Beck, F. Obst, M. Busek, S. Grünzner, P.J. Mehner, G. Paschew, D. Appelhans, B. Voit, A. Richter, Micromachines (Basel) 11, 479 (2020)
A. Negro, T. Cherbuin, M.P. Lutolf, Sci. Rep. 8, 17099 (2018)
T. Schmidt, J.I. Mönch, K.F. Arndt, Macromol. Mater. Eng. 291, 755 (2006)
M.M. Coronel, K.E. Martin, M.D. Hunckler, P. Kalelkar, R.M. Shah, A.J. García, Small 18, 2106896 (2022)
V. Yusupov, S. Churbanov, E. Churbanova, K. Bardakova, A. Antoshin, S. Evlashin, P. Timashev, N. Minaev, Int. J. Bioprint. 6, 271 (2020)
T. Li, Z. Ye, Y. Cai, T. Tu, B. Zhang, S. Zhang, L. Fang, X. Mao, S. Xu, X. Ye, B. Liang, J. Electroanal. Chem. 911, 116183 (2022)
D.G. Tamay, T. Dursun Usal, A.S. Alagoz, D. Yucel, N. Hasirci, V. Hasirci, Front. Bioeng. Biotechnol. 7, 164 (2019). https://doi.org/10.3389/fbioe.2019.00164
S. Miao, N. Castro, M. Nowicki, L. Xia, H. Cui, X. Zhou, W. Zhu, S. Lee, K. Sarkar, G. Vozzi, Y. Tabata, J. Fisher, L.G. Zhang, Mater. Today 20, 577 (2017)
P. Sanjuan-Alberte, C. Whitehead, J.N. Jones, J.C. Silva, N. Carter, S. Kellaway, R.J.M. Hague, J.M.S. Cabral, F.C. Ferreira, L.J. White, F.J. Rawson, iScience. 25, 104552 (2022)
J. Li, C. Wu, P.K. Chu, M. Gelinsky, Mater. Sci. Eng. R Rep. 140, 100543 (2020)
J.A. Lewis, Adv. Funct. Mater. 16, 2193 (2006)
I. Zein, D.W. Hutmacher, K.C. Tan, S.H. Teoh, Biomaterials 23, 1169 (2002)
B. Derby, Annu. Rev. Mater. Res. 40, 395 (2010)
M. Singh, H.M. Haverinen, P. Dhagat, G.E. Jabbour, Adv. Mater. 22, 673 (2010)
R. Suntornnond, W.L. Ng, X. Huang, C.H.E. Yeow, W.Y. Yeong, J. Mater. Chem. B 10, 5989 (2022)
K. Pataky, T. Braschler, A. Negro, P. Renaud, M.P. Lutolf, J. Brugger, Adv. Mater. 24, 391 (2012)
N. Adly, S. Weidlich, S. Seyock, F. Brings, A. Yakushenko, A. Offenhäusser, B. Wolfrum, NPJ Flex. Electron. 2(1), 15 (2018)
H. Najaf Zadeh, D. Bowles, T. Huber, D. Clucas, Materials (Basel) 14, 6988 (2021)
N. Pandala, M.A. LaScola, Y. Tang, M. Bieberich, L.T.J. Korley, E. Lavik, ACS Biomater. Sci. Eng. 7, 5007 (2021)
A. Canales, X. Jia, U.P. Froriep, R.A. Koppes, C.M. Tringides, J. Selvidge, C. Lu, C. Hou, L. Wei, Y. Fink, P. Anikeeva, Nat. Biotechnol. 33, 277 (2015)
S. Park, Y. Guo, X. Jia, H.K. Choe, B. Grena, J. Kang, J. Park, C. Lu, A. Canales, R. Chen, Y.S. Yim, G.B. Choi, Y. Fink, P. Anikeeva, Nat. Neurosci. 20, 612 (2017)
H. Yuk, T. Zhang, S. Lin, G.A. Parada, X. Zhao, Nat. Mater. 15, 190 (2016)
Z. Wang, L. Chen, Y. Chen, P. Liu, H. Duan, P. Cheng, Research 2020, 1426078 (2020). https://doi.org/10.34133/2020/1426078
P. Serra, A. Piqué, Adv. Mater. Technol. 4, 1800099 (2019)
P. Delaporte, A.P. Alloncle, Opt. Laser Technol. 78, 33 (2016)
J. Torgersen, X.H. Qin, Z. Li, A. Ovsianikov, R. Liska, J. Stampfl, Adv. Funct. Mater. 23, 4542 (2013)
A. Accardo, M.-C. Blatché, R. Courson, I. Loubinoux, C. Vieu, L. Malaquin, Biomed. Phys. Eng. Express 4, 027009 (2018)
O. Geuli, N. Metoki, N. Eliaz, D. Mandler, Adv. Funct. Mater. 26, 8003 (2016)
Q. Chen, U.P. de Larraya, N. Garmendia, M. Lasheras-Zubiate, L. Cordero-Arias, S. Virtanen, A.R. Boccaccini, Colloids Surf. B 118, 41 (2014)
A.C. Da Silva, J. Wang, I.R. Minev, Nat. Commun. 13, 1353 (2022)
K. Wang, L. Tian, T. Wang, Z. Zhang, X. Gao, L. Wu, B. Fu, X. Liu, Compos. Interfaces 26, 27 (2019)
H. Yuk, J. Wu, X. Zhao, Nat. Rev. Mater. 7, 935 (2022)
Q. Yang, Z. Hu, J.A. Rogers, Acc. Mater. Res. 2, 1010 (2021)
H.-L. Tan, S.-Y. Teow, J. Pushpamalar, Bioengineering (Basel) 6(1), 17 (2019)
C. Desfrançois, R. Auzély, I. Texier, Pharmaceuticals (Basel) 11(4), 118 (2018)
Q. Shi, H. Liu, D. Tang, Y. Li, X. Li, F. Xu, NPG Asia Mater. 11, 64 (2019)
C.M. Tringides, N. Vachicouras, I. de Lázaro, H. Wang, A. Trouillet, B.R. Seo, A. Elosegui-Artola, F. Fallegger, Y. Shin, C. Casiraghi, K. Kostarelos, S.P. Lacour, D.J. Mooney, Nat. Nanotechnol. 16, 1019 (2021)
J. Zhang, L. Wang, Y. Xue, I.M. Lei, X. Chen, P. Zhang, C. Cai, X. Liang, Y. Lu, J. Liu, Adv. Mater. 35, 2209324 (2023). https://doi.org/10.1002/adma.202209324
Y. Hong, F. Zhou, Y. Hua, X. Zhang, C. Ni, D. Pan, Y. Zhang, D. Jiang, L. Yang, Q. Lin, Y. Zou, D. Yu, D.E. Arnot, X. Zou, L. Zhu, S. Zhang, H. Ouyang, Nat. Commun. 10, 2060 (2019)
S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding, X. Chen, Adv. Sci. 5, 1700527 (2018)
D. Su, X. Bai, X. He, Eur. Polym. J. 181, 111665 (2022)
K. Kim, J.H. Ryu, M.Y. Koh, S.P. Yun, S. Kim, J.P. Park, C.W. Jung, M.S. Lee, H.-I. Seo, J.H. Kim, H. Lee, Sci. Adv. 7, eabc9992 (2021)
X. Luo, Y. Liu, R. Qin, F. Ao, X. Wang, H. Zhang, M. Yang, X. Liu, Appl. Mater. Today 29, 101576 (2022)
Q. Dong, X. Zhong, Y. Zhang, B. Bao, L. Liu, H. Bao, C. Bao, X. Cheng, L. Zhu, Q. Lin, Carbohydr. Polym. 245, 116525 (2020)
D. De Meo, G. Ceccarelli, G. Iaiani, F. Lo Torto, D. Ribuffo, P. Persiani, C. Villani, Gels (Basel) 7, 126 (2021)
M. Gori, S.M. Giannitelli, G. Vadalà, R. Papalia, L. Zollo, M. Sanchez, M. Trombetta, A. Rainer, G. Di Pino, V. Denaro, Molecules 27(10), 3126 (2022)
D. Dong, C. Tsao, H.C. Hung, F. Yao, C. Tang, L. Niu, J. Ma, J. MacArthur, A. Sinclair, K. Wu, P. Jain, M.R. Hansen, D. Ly, S.G. Tang, T.M. Luu, P. Jain, S. Jiang, Sci. Adv. 7, eabc5442 (2021)
F. Robotti, I. Sterner, S. Bottan, J.M. Monné Rodríguez, G. Pellegrini, T. Schmidt, V. Falk, D. Poulikakos, A. Ferrari, C. Starck, Biomaterials 229, 119583 (2020)
F. Horkay, P.J. Basser, Soft Matter 18, 4414 (2022)
S. Peressotti, G.E. Koehl, J.A. Goding, R.A. Green, ACS Biomater. Sci. Eng. 7, 4136 (2021)
Acknowledgments
This work was supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement No. 754354, InnoSuisse Grant 45944.1 IP-ENG, and EPFL Science Seed Fund.
Funding
Open access funding provided by EPFL Lausanne.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sagdic, K., Fernández-Lavado, E., Mariello, M. et al. Hydrogels and conductive hydrogels for implantable bioelectronics. MRS Bulletin 48, 495–505 (2023). https://doi.org/10.1557/s43577-023-00536-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-023-00536-1