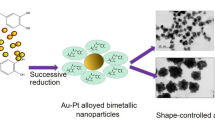
Abstract
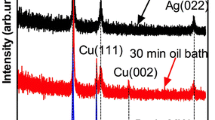
Bimetallic nanoparticles (NPs), particularly Au/Pd and Au/Pt, have attracted extensive attention due to their wide-spread application in catalysis, optoelectronics and energy recuperation.[1] Here we have attempted the fabrication of Au/Pt and Au/Pd bimetallic NPs by an energy-efficient eco-friendly microwave methodology. The microwave-assisted reactions enable considerably large product yields over conventional colloidal methods due to (a) almost two-fold increased reaction kinetics, (b) localized superheating at reaction sites and rapid rise of initial temperature.[2] Au NPs (sizes 20 ± 3 nm) are fabricated in the first step followed by the reduction of [PdCl2(NH3)2] or [K2PtCl6]in tetraethylene glycol at 180 ºC for 2 min. Controlling and understanding the atomic structure and elemental distributions of these NPs are crucial for their optimized performances. So, we address the fundamental question of the most likely arrangement of Au and Pd or Pt atoms in these bimetallic NPs prepared under similar conditions by complementary characterizations using UV-Vis spectroscopy, X-ray diffraction (XRD) and transmission electron microscopy (TEM). The UV-Vis spectroscopy reveals the formation of an alloy shell. The extent of depression of the plasmon peak of Au and its blue-shift reveals substantial deposition of Pd atoms on an Au core and significant alloying in comparison to Au/Pt NPs. XRD reveals the gradual shift of the diffraction peak from the position of Au to the position of Pd or Pt with change in composition. XRD supports the formation of a thick alloy shell in these NPs. However, the TEM images reveal a very interesting result. With increase in Pt concentration, the size of the dispersed NPs decreases from 20 ± 3 nm to about 16 nm (± 1 nm) and there is evolution of a bimodal particle size distribution with small particles about 1-2 nm diameters. On the contrary, with increasing Pd concentration, the particle size of the dispersed particles increases to about 32 nm (± 1 nm). This discrepancy of particle size evolution for the two systems arises due to the differences in surface energies (Pt > Pd > Au atoms). Pt atoms tend to diffuse towards the core with the formation of Au nano-islands which eventually segregates leading to a reduction in particle size and bimodal distribution. At higher concentration of Pt, Pt and Au atoms tend to nucleate separately also contribute to the bimodal distribution. While for Au/Pd NPs, we have an Au core with an alloyed shell having higher Pd concentration. This is further supported by experimental evidence by selective etching and dissolution of Au by potassium-iodide solution. Furthermore, the Au/Pd bimetallic NPs are found to possess better catalytic activities in the reduction of 4-nitrophenol to 4-aminophenol than Au/Pt and monometallic NPs.
Similar content being viewed by others
References
R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev. 108, 845 (2008).
N. Dahal, S. García, J. Zhou and S. M. Humphrey, ACS Nano 6, 9433 (2012).
M. Sankar, N. Dimitratos, P. J. Miedziak, P. P. Wells, C. J. Kielye and G. J. Hutchings, Chem. Soc. Rev. 41, 8099 (2012).
A. Roucoux, J. Schulz and H. Patin, Chem. Rev. 102, 3757 (2002).
F. Gao and D. W. Goodman, Chem. Soc. Rev. 41, 8009 (2012).
C. J. Serpell, J. Cookson, D. Ozkaya and P. D. Beer, Nature Chem. 3, 478 (2011).
M. Baghbanzadeh, L.Carbone, P. D. Cozzoli and C. O. Kappe, Angew. Chem. Int. Ed. 50, 11312 (2011).
C. Kan, W. Cai, C. Li, L. Zhang and H. Hofmeister, J. Phys. D: Appl. Phys. 36, 1609 (2003).
S. Link and M. A. El-Sayed, Int. Rev. Phys. Chem. 18, 409 (2000).
L. Vitos, A.V. Ruban, H. L. Skriver, J. Kollár, Surf. Sci. 411, 186 (1998).
B. N. Wanjala, J. Luo, B. Fang, D. Mott and C. –J. Zhong. Mater. Chem. 21, 4012 (2011).
X. Zhang, J. Yan, S. Han, H. Shioyama and Q. Xu, J. Am. Chem. Soc. 131, 2779 (2009).
P. Hervés, M. Pérez-Lorenzo, L. M. Liz-Marzán, J. Dzubiella, Y. Lu and M. Ballauff, Chem. Soc. Rev. 41, 5577 (2012).
T. Som, G. V. Troppenz, R. Wendt, M. Wollgarten, J. Rappich, F. Emmerling and K. Rademann, ChemSusChem 7, 854 (2014).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Som, T., Wendt, R., Raoux, S. et al. Structural Evolution of AuPt and AuPd Nanoparticles Fabricated by Microwave Assisted Synthesis: A Comparative Study. MRS Online Proceedings Library 1802, 2 (2015). https://doi.org/10.1557/opl.2015.383
Published:
DOI: https://doi.org/10.1557/opl.2015.383