Abstract
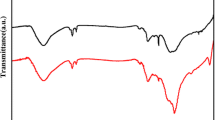
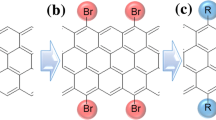
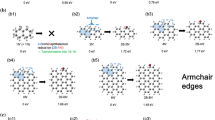
Developing easy and effective surface functionalization approaches has required to facilitate the processability of graphene while seeking novel application areas. Herein, an in situ single-step reductive covalent bromination of graphene has been reported for the first time. Highly brominated graphene flakes (>3% Br) were prepared by only subjecting the bromine-intercalated graphite flakes to a reduction reaction with reactive lithium naphthalide. The bromine-functionalized graphene was characterized by X-ray photoelectron spectroscopy and thermogravimetric analysis. Results revealed that Br2 molecules acted as both an intercalating agent for the graphite and a reactant for the surface functionalization of the graphene. After brominating, the remaining negative charges on the reduced graphene surface were further used for the dual surface functionalization of graphene with a long-chain alkyl group (∼1% dodecyl group addition). The functionalized graphenes were also characterized by Fourier transform infrared and Raman spectroscopy.






Similar content being viewed by others
References
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, and A.A. Firsov: Electric field effect in atomically thin carbon films. Science 306, 666 (2004).
K.S. Novoselov, D. Jiang, F. Schedin, T.J. Booth, V.V. Khotkevich, S.V. Morozov, and A.K. Geim: Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. U.S.A. 102, 10451 (2005).
Y. Hernandez, V. Nicolosi, M. Lotya, F.M. Blighe, Z. Sun, S. De, I.T. McGovern, B. Holland, M. Byrne, Y.K. Gun’Ko, J.J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A.C. Ferrari, and J.N. Coleman: High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 3, 563 (2008).
M. Lotya, Y. Hernandez, P.J. King, R.J. Smith, V. Nicolosi, L.S. Karlsson, F.M. Blighe, S. De, Z. Wang, I.T. McGovern, G.S. Duesberg, and J.N. Coleman: Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131, 3611 (2009).
D.W. Johnson, B.P. Dobson, and K.S. Coleman: A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 20, 367 (2015).
K.R. Paton, E. Varrla, C. Backes, R.J. Smith, U. Khan, A. O’Neill, C. Boland, M. Lotya, O.M. Istrate, P. King, T. Higgins, S. Barwich, P. May, P. Puczkarski, I. Ahmed, M. Moebius, H. Pettersson, E. Long, J. Coelho, S.E. O’Brien, E.K. McGuire, B.M. Sanchez, G.S. Duesberg, N. McEvoy, T.J. Pennycook, C. Downing, A. Crossley, V. Nicolosi, and J.N. Coleman: Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 13, 624 (2014).
K. Parvez, Z-S. Wu, R. Li, X. Liu, R. Graf, X. Feng, and K. Müllen: Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 136, 6083 (2014).
S.A. Hodge, M.K. Bayazit, K.S. Coleman, and M.S.P. Shaffer: Unweaving the rainbow: A review of the relationship between single-walled carbon nanotube molecular structures and their chemical reactivity. Chem. Soc. Rev. 41, 4409 (2012).
M.K. Bayazit and K.S. Coleman: Probing the selectivity of azomethine imine cycloaddition to single-walled carbon nanotubes by resonance Raman spectroscopy. Chem. - Asian J. 7, 2925 (2012).
M.K. Bayazit and J. Tang: Graphene production method WO/2019/110757, UCL Business PLC, UK, 2019. p. 46pp.
M.K. Bayazit and K.S. Coleman: Ester-functionalized single-walled carbon nanotubes via addition of haloformates. J. Mater. Sci. 49, 5190 (2014).
A.S. Jombert, M.K. Bayazit, C.R. Herron, K.S. Coleman, and D.A. Zeze: Synthesis and characterization of molecularly-bridged single-walled carbon nanotubes and electrical properties of their films. Sci. Adv. Mater. 5, 1967 (2013).
M.K. Bayazit, A. Suri, and K.S. Coleman: Formylation of single-walled carbon nanotubes. Carbon 48, 3412 (2010).
M.K. Bayazit, L.S. Clarke, K.S. Coleman, and N. Clarke: Pyridine-functionalized single-walled carbon nanotubes as gelators for poly(acrylic acid) hydrogels. J. Am. Chem. Soc. 132, 15814 (2010).
M.K. Bayazit and K.S. Coleman: Fluorescent single-walled carbon nanotubes following the 1,3-dipolar cycloaddition of pyridinium ylides. J. Am. Chem. Soc. 131, 10670 (2009).
G. Bottari, M.Á. Herranz, L. Wibmer, M. Volland, L. Rodríguez-Pérez, D.M. Guldi, A. Hirsch, N. Martín, F. D’Souza, and T. Torres: Chemical functionalization and characterization of graphene-based materials. Chem. Soc. Rev. 46, 4464 (2017).
W.S. Hummers and R.E. Offeman: Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958).
K.R. Nandanapalli, D. Mudusu, and S. Lee: Functionalization of graphene layers and advancements in device applications. Carbon 152, 954 (2019).
Z.B. Lei, J.T. Zhang, L.L. Zhang, N.A. Kumar, and X.S. Zhao: Functionalization of chemically derived graphene for improving its electrocapacitive energy storage properties. Energy Environ. Sci. 9, 1891 (2016).
A. Narita, X.Y. Wang, X.L. Feng, and K. Mullen: New advances in nanographene chemistry. Chem. Soc. Rev. 44, 6616 (2015).
J. Greenwood, T.H. Phan, Y. Fujita, Z. Li, O. Lvasenko, W. Vanderlinden, H. Van Gorp, W. Frederickx, G. Lu, K. Tahara, Y. Tobe, H. Uji-i, S.F.L. Mertens, and S. De Feyter: Covalent modification of graphene and graphite using diazonium chemistry: Tunable grafting and nanomanipulation. ACS Nano 9, 5520 (2015).
G.L.C. Paulus, Q.H. Wang, and M.S. Strano: Covalent electron transfer chemistry of graphene with diazonium salts. Acc. Chem. Res. 46, 160 (2013).
J.E. Johns and M.C. Hersam: Atomic covalent functionalization of graphene. Acc. Chem. Res. 46, 77 (2013).
L.M. Dai: Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res. 46, 31 (2013).
T. Kuila, S. Bose, A.K. Mishra, P. Khanra, N.H. Kim, and J.H. Lee: Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 57, 1061 (2012).
S. Niyogi, E. Bekyarova, J. Hong, S. Khizroev, C. Berger, W. de Heer, and R.C. Haddon: Covalent chemistry for graphene electronics. J. Phys. Chem. Lett. 2, 2487 (2011).
M. Quintana, K. Spyrou, M. Grzelczak, W.R. Browne, P. Rudolf, and M. Prato: Functionalization of graphene via 1,3-dipolar cycloaddition. ACS Nano 4, 3527 (2010).
E. Bekyarova, M.E. Itkis, P. Ramesh, C. Berger, M. Sprinkle, W.A. de Heer, and R.C. Haddon: Chemical modification of epitaxial graphene: Spontaneous grafting of aryl groups. J. Am. Chem. Soc. 131, 1336 (2009).
V. Georgakilas: Covalent attachment of organic functional groups on pristine graphene. In Functionalization of Graphene, V. Georgakilas, ed. (Wiley, Germany 2014); p. 21.
J.M. Englert, C. Dotzer, G.A. Yang, M. Schmid, C. Papp, J.M. Gottfried, H.P. Steinruck, E. Spiecker, F. Hauke, and A. Hirsch: Covalent bulk functionalization of graphene. Nat. Chem. 3, 279 (2011).
Z. Jin, T.P. McNicholas, C.J. Shih, Q.H. Wang, G.L.C. Paulus, A. Hilmer, S. Shimizu, and M.S. Strano: Click chemistry on solution-dispersed graphene and monolayer CVD graphene. Chem. Mater. 23, 3362 (2011).
F.M. Koehler, A. Jacobsen, K. Ensslin, C. Stampfer, and W.J. Stark: Selective chemical modification of graphene surfaces: Distinction between single- and bilayer graphene. Small 6, 1125 (2010).
M. Fang, K.G. Wang, H.B. Lu, Y.L. Yang, and S. Nutt: Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 19, 7098 (2009).
A.J. Clancy, M.K. Bayazit, S.A. Hodge, N.T. Skipper, C.A. Howard, and M.S.P. Shaffer: Charged carbon nanomaterials: Redox chemistries of fullerenes, carbon nanotubes, and graphenes. Chem. Rev. 118, 7363 (2018).
A. Pénicaud and C. Drummond: Deconstructing graphite: Graphenide solutions. Acc. Chem. Res. 46, 129 (2013).
R.A. Schäfer, J.M. Englert, P. Wehrfritz, W. Bauer, F. Hauke, T. Seyller, and A. Hirsch: On the way to graphane—Pronounced fluorescence of polyhydrogenated graphene. Angew. Chem., Int. Ed. 52, 754 (2013).
K.C. Knirsch, J.M. Englert, C. Dotzer, F. Hauke, and A. Hirsch: Screening of the chemical reactivity of three different graphite sources using the formation of reductively alkylated graphene as a model reaction. Chem. Commun. 49, 10811 (2013).
T. Morishita, A.J. Clancy, and M.S.P. Shaffer: Optimised exfoliation conditions enhance isolation and solubility of grafted graphenes from graphite intercalation compounds. J. Mater. Chem. A 2, 15022 (2014).
F. Hof, R.A. Schäfer, C. Weiss, F. Hauke, and A. Hirsch: Novel λ3-iodane-based functionalization of synthetic carbon allotropes (SCAs)—Common concepts and quantification of the degree of addition. Chem. Eur J. 20, 16644 (2014).
D. Voiry, O. Roubeau, and A. Pénicaud: Stoichiometric control of single walled carbon nanotubes functionalization. J. Mater. Chem. 20, 4385 (2010).
A.J. Clancy, P. Sirisinudomkit, D.B. Anthony, A.Z. Thong, J.L. Greenfield, M.K. Salaken Singh, and M.S.P. Shaffer: Real-time mechanistic study of carbon nanotube anion functionalisation through open circuit voltammetry. Chem. Sci. 10, 3300 (2019).
H. Au, N. Rubio, and M.S.P. Shaffer: Brominated graphene as a versatile precursor for multifunctional grafting. Chem. Sci. 9, 209 (2018).
O. Jankovský, P. Šimek, K. Klimová, D. Sedmidubský, S. Matějková, M. Pumera, and Z. Sofer: Towards graphene bromide: Bromination of graphite oxide. Nanoscale 6, 6065 (2014).
M.S. Dresselhaus and G. Dresselhaus: Intercalation compounds of graphite. Adv. Phys. 51, 1 (2002).
P.C. Eklund, N. Kambe, G. Dresselhaus, and M.S. Dresselhaus: In-plane intercalate lattice modes in graphite-bromine using Raman spectroscopy. Phys. Rev. B 18, 7069 (1978).
C. Underhill, S.Y. Leung, G. Dresselhaus, and M.S. Dresselhaus: Infrared and Raman spectroscopy of graphite-ferric chloride. Solid State Commun. 29, 769 (1979).
J. Xu, Y. Dou, Z. Wei, J. Ma, Y. Deng, Y. Li, H. Liu, and S. Dou: Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-ion batteries. Adv. Sci. 4, 1700146 (2017).
J. Zou, C. Sole, N.E. Drewett, M. Velický, and L.J. Hardwick: In situ study of Li intercalation into highly crystalline graphitic flakes of varying thicknesses. J. Phys. Chem. Lett. 7, 4291 (2016).
T. Sasa, Y. Takahashi, and T. Mukaibo: Crystal structure of graphite bromine lamellar compounds. Carbon 9, 407 (1971).
W.T. Eeles, J.A. Turnbull, and L. Rotherham: The crystal structure of graphite-bromine compounds. Proc. R. Soc. London, Ser. A 283, 179 (1965).
S. Pekker, J.P. Salvetat, E. Jakab, J.M. Bonard, and L. Forró: Hydrogenation of carbon nanotubes and graphite in liquid ammonia. J. Phys. Chem. B 105, 7938 (2001).
K. Nemeth, E. Jakab, F. Borondics, H.M. Tóháti, Á. Pekker, M. Bokor, T. Verebélyi, K. Tompa, S. Pekker, and K. Kamarás: Breakdown of diameter selectivity in a reductive hydrogenation reaction of single-walled carbon nanotubes. Chem. Phys. Lett. 618, 214 (2015).
A.C. Ferrari and D.M. Basko: Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 8, 235 (2013).
G. Das, B.J. Park, J. Kim, D. Kang, and H.H. Yoon: Quaternized cellulose and graphene oxide crosslinked polyphenylene oxide based anion exchange membrane. Sci. Rep. 9, 9572 (2019).
J.S. Culik and D.D.L. Chung: Thermal gravimetric analysis of graphite-bromine compounds. Mater. Sci. Eng. 44, 129 (1980).
M.K. Bayazit, S.A. Hodge, A.J. Clancy, R. Menzel, S. Chen, and M.S.P. Shaffer: Carbon nanotube anions for the preparation of gold nanoparticle–nanocarbon hybrids. Chem. Commun. 52, 1934 (2016).
Acknowledgments
We gratefully acknowledge the Sabanci University Nanotechnology Research and Application Center (I.A.SN-19-00004) for research funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayazit, M.K. In situ single-step reduction of bromine-intercalated graphite to covalently brominated and alkylated/brominated graphene. Journal of Materials Research 35, 1472–1480 (2020). https://doi.org/10.1557/jmr.2020.112
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2020.112