Abstract
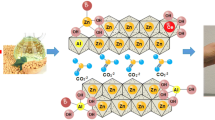
ZnAl–Zr(X) hydrotalcite-like materials were synthesized by co-precipitation using a Zn/Al molar ratio of 2 and Zr/Al(X) molar ratios of 0.0, 0.10, and 0.25. The effect of the activation temperature on the catalytic performance of these materials was analyzed, revealing that at relatively low temperature (200 °C), the collapse of the material structure is diminished, leading to FAME yields varying from 68 to 82%. This remarkable catalytic activity is related to the formation of hydrotalcite, zincite, and hydrozincite which in turn lead to the generation of Brönsted basic sites and Lewis acid–basic pairs. Incorporation of Zr+4 into the brucite-like structure of hydrotalcites enhances the basicity of ZnAl–Zr(X) catalysts, which correlates well with the increase in catalytic activity observed for these catalysts. The stability of the ZnAl–Zr(0.25) catalyst was further studied, showing insignificant deactivation after five subsequent reaction cycles. A simplified reaction scheme was proposed for the transesterification reaction over these materials.







Similar content being viewed by others
References
A. Datta and B.K. Mandal: A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renewable Sustainable Energy Rev. 57, 799 (2016).
J. Thangaraja, K. Anand, and P.S. Mehta: Biodiesel NOx penalty and control measures—A review. Renewable Sustainable Energy Rev. 61, 1 (2016).
M. Tariq, S. Ali, and N. Khalid: Activity of homogeneous and heterogeneous catalysts, spectroscopic and chromatographic characterization of biodiesel: A review. Renewable Sustainable Energy Rev. 16, 6303 (2012).
Y.C. Sharma, B. Singh, and J. Korstad: Latest developments on application of heterogeneous basic catalysts for an efficient and eco-friendly synthesis of biodiesel: A review. Fuel 90, 1309 (2011).
M. Sasidharan and R. Kumar: Transesterification over various zeolites under liquid-phase conditions. J. Mol. Catal. A: Chem. 210, 93 (2004).
M.J. Ramos, A. Casas, L. Rodríguez, R. Romero, and A. Pérez: Transesterification of sunflower oil over zeolites using different metal loading: A case of leaching and agglomeration studies. Appl. Catal., A 346, 79 (2008).
S.L. Martínez, R. Romero, J.C. López, A. Romero, V. Sánchez Mendieta, and R. Natividad: Preparation and characterization of CaO nanoparticles/NaX zeolite catalysts for the transesterification of sunflower oil. Ind. Eng. Chem. Res. 50, 2665 (2011).
T.F. Dossin, M-F. Reyniers, and G.B. Marin: Kinetics of heterogeneously MgO-catalyzed transesterification. Appl. Catal., B 61, 35 (2006).
X. Liu, H. He, Y. Wang, S. Zhu, and X. Piao: Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 87, 216 (2008).
A. Galadima and O. Muraza: Biodiesel production from algae by using heterogeneous catalysts: A critical review. Energy 78, 72 (2014).
H. Sun, Y. Ding, J. Duan, Q. Zhang, Z. Wang, H. Lou, and X. Zheng: Transesterification of sunflower oil to biodiesel on ZrO2 supported La2O3 catalyst. Bioresour. Technol. 101, 953 (2010).
R. Madhuvilakku and S. Piraman: Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 150, 55 (2013).
P. Hernández-Hipólito, M. García-Castillejos, E. Martínez-Klimova, N. Juárez-Flores, A. Gómez-Cortés, and T. Klimova: Biodiesel production with nanotubular sodium titanate as a catalysts. Catal. Today 220–222, 4 (2014).
Y. Liu, E. Lotero, J.G. Goodwin, Jr., and X. Mo: Transesterification of poultry fat with methanol using Mg–Al hydrotalcite derived catalysts. Appl. Catal., A 331, 138 (2007).
H-Y. Zeng, K-B. Liao, X. Deng, H. Jiang, and F. Zhang: Characterization of the lipase immobilized on Mg–Al hydrotalcite for biodiesel. Process Biochem. 444, 791 (2009).
C. Sun, F. Qiu, D. Yang, and B. Ye: Preparation of biodiesel from soybean oil catalyzed by Al–Ca hydrotalcite loaded with K2CO3 as heterogeneous solid base catalyst. Fuel Process. Technol. 126, 383 (2014).
J. Nowicki, J. Lach, M. Organek, and E. Sabura: Transesterification of rapeseed oil to biodiesel over Zr-dopped MgAl hydrotalcites. Appl. Catal., A 524, 17 (2016).
W. Jiang, H.F. Lu, T. Qi, S.L. Yan, and B. Liang: Preparation, application, and optimization of Zn/Al complex oxides for biodiesel production under sub-critical conditions. Biotechnol. Adv. 28, 620 (2010).
F. Tzompantzi, Y. Carrera, G. Morales-Mendoza, G. Valverde-Aguilar, and A. Mantilla: ZnO–Al2O3–La2O3 layered double hydroxides as catalyst precursors for the esterification of oleic acid fatty grass at low temperature. Catal. Today 212, 164 (2013).
A.P. Soares Dias, J. Bernardo, P. Felizardo, and M.J. Neiva Correia: Biodiesel production over thermal activated cerium modified Mg–Al hydrotalcites. Energy 41, 344 (2012).
Q. Liu, C. Wang, W. Qu, B. Wang, Z. Tian, H. Ma, and R. Xu: The application of Zr incorporated Zn–Al dehydrated hydrotalcites as solid base in transesterification. Catal. Today 234, 161 (2014).
F. Cavani, F. Trifiró, and A. Vaccari: Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 11, 173 (1991).
D.A. Cabrera-Munguia, F. Tzompantzi, A. Gutiérrez-Alejandre, J.L. Rico, and H. González: ZnAl–Zr hydrotalcite-like compounds activated at low temperature as solid base catalyst for the transesterification of vegetable oils. Energy Procedia 142, 582 (2017).
J.M. Fraile, N. García, J.A. Mayoral, E. Pires, and L. Roldán: The basicity of mixed oxides and the influence of alkaline metals: The case of transesterification reactions. Appl. Catal., A 387, 67 (2010).
D.A. Cabrera-Munguía, H. González, A. Gutiérrez-Alejandre, J.L. Rico, R. Huirache-Acuña, R. Maya-Yescas, and R.E. del Río: Heterogeneous acid conversion of a tricaprylin-palmitic acid mixture over Al-SBA-15 catalysts: Reaction study for biodiesel synthesis. Catal. Today 282, 195 (2017).
S. Velu, D.P. Sabde, N. Shah, and S. Sivasanker: New hydrotalcite-like anionic clays containing Zr4+ in the layers: Synthesis and physicochemical properties. Chem. Mater. 10, 3451 (1998).
D. Tichit, N. Das, B. Coq, and R. Durand: Preparation of Zr-containing layered double hydroxides and characterization of the acid-basic properties of their mixed oxides. Chem. Mater. 14, 1530 (2002).
P. Koilraj and S. Kannan: Phosphate uptake behavior of ZnAlZr ternary layered double hydroxides through surface precipitation. J. Colloid Interface Sci. 341, 289 (2010).
N.N. Das, J. Konar, M.K. Mohanta, and S.C. Srivastava: Adsorption of Cr(VI) and Se(IV) from their aqueos solutions onto Zr4+-substituted ZnAl/MgAl-layered double hydroxides: Effect of Zr4+ substitution in the layer. J. Colloid Interface Sci. 270, 1 (2004).
E.M. Seftel, E. Popovici, M. Mertens, K. De Witte, G. Van Tendeloo, P. Cool, and E.F. Vansant: Zn–Al layered double hydroxides: Synthesis, characterization and photocatalytic application. Microporous Mesoporous Mater. 113, 296 (2008).
J.S. Valente, H. Pfeiffer, E. Lima, J. Prince, and J. Flores: Cyanoethylation of alcohols by activated Mg–Al layered double hydroxides: Influence of rehydration conditions and Mg/Al molar ratio on Brönsted basicity. J. Catal. 279, 196 (2011).
M.G. Álvarez, R.J. Chimentᾶo, N. Barrabés, K. Föttinger, F. Gispert-Guirado, E. Kleymenov, D. Tichit, and F. Medina: Structure evolution of layered double hydroxides by ultrasound induced reconstruction. Appl. Clay Sci. 83–84, 1 (2013).
D. Wu, W. Wang, F. Tan, F. Sun, H. Lu, and X. Qiao: Fabrication of pit-structures ZnO nanorods and their enhanced photocatalytic performance. RSC Adv. 3, 20054 (2013).
T. López, E. Ramos, P. Bosch, M. Asomoza, and R. Gómez: DTA and TGA characterization of sol–gel hydrotalcites. Mater. Lett. 30, 279 (1997).
C. Vaysse, L. Guerlou-Demorgues, and C. Delmas: Thermal evolution of carbonate pillared layered hydroxides with (Ni, L)(L = Fe, Co) base slabs: Grafting or non grafting of carbonate anions? Inorg. Chem. 41, 6905 (2001).
P.M. Veiga, A.S. Luna, M.F. Portilho, C.O. Veloso, and C.A. Henriques: Zn, Al-catalysts for heterogeneous biodiesel production: Basicity and process optimization. Energy 75, 453 (2014).
Q. Liu, C. Wang, W. Qu, B. Wang, Z. Tian, H. Ma, and R. Xu: Basicities and transesterification activities of Zn–Al hydrotalcites-derived solid bases. Green Chem. 16, 2604 (2014).
G. Busca, P.F. Rossi, V. Lorenzelli, V. Benaissa, J. Travert, and J.C. Lavalley: Microcalorimetric and Fourier transform infrared spectroscopic studies of methanol adsorption on alumina. J. Phys. Chem. 89, 5433 (1985).
A. Riva, F. Trifiro, A. Vaccari, L. Mintchev, and G. Busca: Structure and reactivity of zinc–chromium mixed oxides. Part 2: Study of the surface reactivity by temperature-programmed desorption of methanol. J. Chem. Soc., Faraday Trans. 84, 1423 (1988).
C. Chauvin, J. Saussey, J.C. Lavalley, H. Idriss, J.P. Hindermann, A. Kiennemann, P. Chaumette, and P. Courty: Combined infrared spectroscopy, chemical trapping, and thermos programmed desorption studies of methanol and decomposition on ZnAl2O4 and Cu/ZnAl2O4. J. Catal. 121, 56 (1990).
T. Montanari, M. Sisani, M. Nocchetti, R. Vivani, M.C. Herrera-Delgado, G. Ramis, G. Busca, and U. Constantino: Zinc–aluminum hydrotalcites as precursors of basic catalysts: Preparation, characterization and study of the activation of methanol. Catal. Today 152, 104 (2010).
S. Velu, V. Ramkumar, A. Narayanan, and C.S. Swamy: Effect of interlayer anions on the physicochemical properties of zinc–aluminum hydrotalcite-like compunds. J. Mater. Sci. 32, 957 (1997).
F.R. Chen, J.G. Davis, and J.J. Fripiat: Aluminum coordination and lewis acidity in transition aluminas. J. Catal. 133, 263 (1992).
D. Coster and J.J. Fripiat: Memory effects in gel-solid transformations: Coordinately unsaturated Al sites in nanosized aluminas. Chem. Mater. 5, 1204 (1993).
J.A. Wang, X. Bokhimi, O. Novaro, T. López, F. Tzompantzi, R. Gómez, J. Navarrete, M.E. Llanos, and M.E. López-Salinas: Effects of structural defects and acid-basic properties on the activity and selectivity of isopropanol decomposition on nanocrystallite sol–gel alumina catalyst. J. Mol. Catal. A: Chem. 137, 239 (1999).
J.M. Vohs and M.A. Barteau: Photoelectron spectroscopy of diethylzinc on the polar surfaces of zinc oxide. J. Electron Spectrosc. Relat. Phenom. 49, 87 (1989).
P. Stoyanov, S. Akhtert, and J.M. White: XPS study of metal/polymer interaction: Evaporated aluminum on polyvinyl alcohol. Surf. Interface Anal. 15, 509 (1990).
R. Sanna, D. Medas, F. Poddda, C. Meneghini, M. Casu, P. Lattanzi, M.A. Scorciapino, C. Floris, C. Cannas, and G. de Giudici: Binding of bis-(2-ethylhexyl) phthalate at the surface of hydrozincite nanocrystals: An example of organic molecules absorption onto nanocrystalline minerals. J. Colloid Interface Sci. 457, 298 (2015).
A. Dermibas: Biodiesel: A Realistic Fuel Alternative for Diesel Engines (Springer, London, 2008).
ACKNOWLEDGMENT
The authors greatly appreciate financial support by CIC-UMSNH. DACM thanks CONACyT for the grant (487883) received during the development of this work. The authors also thank Rogelio Cuevas García for his assistance with the TGA experiments.
Author information
Authors and Affiliations
Corresponding author
Supplementary Material
Rights and permissions
About this article
Cite this article
Cabrera-Munguía, D.A., González, H., Tzompantzi, F. et al. New insights on the basicity of ZnAl–Zr hydrotalcites activated at low temperature and their application in transesterification of soybean oil. Journal of Materials Research 33, 3614–3624 (2018). https://doi.org/10.1557/jmr.2018.312
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2018.312