Abstract
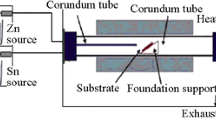
In this study, the authors have comparatively studied the influence of H2 addition on the structures and properties of ZnO films grown by metal organic (MO) chemical vapor deposition with dimethyl zinc and diethyl zinc as zinc precursors and N2O and O2 as oxygen sources, respectively. Various characterization methods, like x-ray diffraction, Raman scattering, Hall effect, photoluminescence, and atomic force microscopy, have been utilized, showing that H2 has different effects on different MO precursors and oxidants. The H2 addition has significantly improved the crystal structural quality of ZnO thin films for the case of dimethyl zinc source, but an opposite effect has been found for the case of diethyl zinc. Moreover, the H2 addition can significantly improve the optical properties of the ZnO films, regardless of the zinc MO sources used, with the surface morphology improved too. The suppression of carbon-related contaminations depends on the use of different precursors and whether H2 is added. By analyzing the experimental results, we have given the effects of H2 on the decomposition of the discussed MO precursors and oxidants, the proposed mechanism could be used in understanding the experimental data.









Similar content being viewed by others
References
P. Zhu, Z.K. Tang, and G.K.L. Wong: Ultraviolet spontaneous and stimulated emissions from ZnO microcrystallite thin films at room temperature. Solid State Commun. 103, 459 (1997).
D.M. Bagnall, Y.F. Chen, Z. Zhu, T. Yao, S. Koyama, M.Y. Shen, and T. Goto: Optically pumped lasing of ZnO at room temperature. Appl. Phys. Lett. 70, 2230 (1997).
J.D. Ye, S.L. Gu, S.M. Zhu, S.M. Liu, W. Liu, X. Zhou, L.Q. Hu, R. Zhang, Y. Shi, and Y.D. Zheng: Comparative study of diethylzinc and dimethylzinc for the growth of ZnO. J. Cryst. Growth 274, 489 (2005).
N. Oleynik, M. Adam, A. Krtschil, J. Blasing, A. Dadgar, F. Bertram, D. Forster, A. Diez, A. Greiling, M. Seip, J. Christen, and A. Krost: Metalorganic chemical vapor phase deposition of ZnO with different O-precursors. J. Cryst. Growth 248, 14 (2003).
D.A. Lamb and S.J.C. Irvine: Growth properties of thin film ZnO deposited by MOCVD with n-butyl alcohol as the oxygen precursor. J. Cryst. Growth 273, 111 (2004).
C.K. Lau, S.K. Tiku, and K.M. Lakin: Growth of epitaxial ZnO thin films by organometallic chemical vapor deposition. J. Electrochem. Soc. 127, 1843 (1980).
S. Oda, H. Tokunaga, N. Kitajima, J. Hanna, I. Shimuzu, and H. Kodado: Highly oriented ZnO films prepared by MOCVD from diethylzinc and alcohols. Jpn. J. Appl. Phys. 24, 1607 (1985).
V.A.L. Roy, A.B. Djurisic, W.K. Chan, J. Gao, H.F. Lui, and C. Surya: Luminescent and structural properties of ZnO nanorods prepared under different conditions. Appl. Phys. Lett. 83, 141 (2003).
C.Y. Leung, A.B. Djurišić, Y.H. Leung, L. Ding, C.L. Yang, and W.K. Ge: Influence of the carrier gas on the luminescence of ZnO tetrapod nanowires. J. Cryst. Growth 290, 131 (2006).
A. Marzouki, A. Sayari, F. Jomard, V. Sallet, A. Lusson, and M. Oueslati: Carrier gas and VI/II ratio effects on carbon clusters incorporation into ZnO films grown by MOCVD. Mater. Sci. Semicond. Process. 16, 1022 (2013).
J.H. Park, D. Byun, B.J. Jeon, and J.K. Lee: Effect of hydrogen content on the ZnO thin films on the surface of polyethylene terephthalate substrate through electron cyclotron resonance-metal organic chemical vapor deposition. J. Mater. Sci. 43, 3471 (2008).
S.J. Baik, J.H. Jang, C.H. Lee, W.Y. Cho, and K.S. Lim: Highly textured and conductive undoped ZnO film using hydrogen post-treatment. Appl. Phys. Lett. 70, 3516 (1997).
S.Y. Myong and K.S. Lim: Highly stable and textured hydrogenated ZnO thin films. Appl. Phys. Lett. 82, 3026 (2003).
S.Y. Myong and K.S. Lim: Alternate deposition and hydrogen doping technique for ZnO thin films. J. Cryst. Growth 293, 253 (2006).
G.Y. Zhu, S.L. Gu, S.M. Zhu, S.M. Huang, R. Gu, J.D. Ye, and Y.D. Zheng: Optimization study of metal-organic chemical vapor deposition of ZnO on sapphire substrate. J. Cryst. Growth 349, 6 (2012).
K. Tang, S.L. Gu, S.M. Zhu, J.G. Liu, H. Chen, J.D. Ye, R. Zhang, and Y.D. Zheng: Suppression of compensation from nitrogen and carbon related defects for p-type N-doped ZnO. Appl. Phys. Lett. 95, 192106 (2009).
W.H. Sun, S.T. Wang, J.C. Zhang, K.M. Chen, G.G. Qin, Y.Z. Tong, Z.J. Yang, G.Y. Zhang, Y.M. Pu, Q.L. Zhang, J. Li, J.Y. Lin, and H.X. Jiang: Formation and dissolution of microcrystalline graphite in carbon-implanted GaN. J. Appl. Phys. 88, 5662 (2000).
A. Marzouki, A. Lusson, F. Jomard, A. Sayari, P. Galtier, M. Oueslati, and V. Sallet: SIMS and Raman characterizations of ZnO: N thin films grown by MOCVD. J. Cryst. Growth 312, 3063 (2010).
J.G. Liu, S.L. Gu, S.M. Zhu, K. Tang, X.D. Liu, H. Chen, and Y.D. Zheng: The influences of O/Zn ratio and growth temperature on carbon impurity incorporation in ZnO grown by metal-organic chemical vapor deposition. J. Cryst. Growth 312, 2710 (2010).
M. Avella, A.C. Prieto, J. Jimenez, A. Rodriguez, J. Sangrador, T. Rodriguez, M.I. Ortiz, and C. Ballesteros: Influence of the crystallization process on the luminescence of multilayers of SiGe nanocrystals embedded in SiO2. Mater. Sci. Eng., B 147, 200 (2008).
K. Tang, S.L. Gu, S.M. Zhu, W. Liu, J.D. Ye, J.M. Zhu, R. Zhang, Y.D. Zheng, and X.W. Sun: Carbon clusters in N-doped ZnO by metal-organic chemical vapor deposition. Appl. Phys. Lett. 93, 132107 (2008).
N. Kobayashi and T. Makimoto: Reduced carbon contamination in OMVPE grown GaAs and AlGaAs. Jpn. J. Appl. Phys. 24, 824 (1985).
M.A. Rueter and J.M. Vohs: Adsorption and reaction of diethylzinc on GaAs(100). J. Vac. Sci. Technol., B 10, 2163 (1992).
G. Klivényi, I. Kovács, and F. Solymosi: Thermal and photo-induced dissociation of (C2H5)2Zn on Rh(111) surface. Surf. Sci. 442, 115 (1999).
ACKNOWLEDGMENTS
This research is supported by the State Key Program for Basic Research of China under Grant No. 2011CB302003 and National Natural Science Foundation of China (Nos. 61025020, 60990312, 61274058, and 61322403), Basic Research Program of Jiangsu Province (BK2011437 and BK20130013), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mao, H., Gu, S., Ye, J. et al. Comparative study of the effect of H2 addition on ZnO films grown by different zinc and oxygen precursors. Journal of Materials Research 30, 935–945 (2015). https://doi.org/10.1557/jmr.2015.81
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2015.81