Abstract
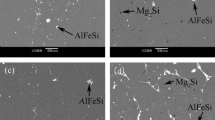
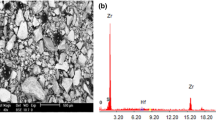
The corrosion behavior of 2099 Al-Li alloy in NaCl aqueous solutions with different concentrations (1.5, 3.5, and 5.0% in mass fraction) was investigated. Its corrosion resistance was evaluated using electrochemical measurements together with full immersion tests. The results showed that the 2099 Al-Li alloy possessed good corrosion resistance in NaCl aqueous solutions. Its corrosion rate increased with increasing chloride ion concentration. The main form of corrosion failure was pitting corrosion. The impurity containing sulfur leads to surface pitting. The oxide films that formed during the manufacturing process offer a good resistance to corrosion. They are likely to suffer separation, cracking, and drop-off when immersed in aggressive NaCl aqueous solution. The good corrosion susceptibility of the alloy may be attributed to homogeneous coherent nanoscale precipitates.










Similar content being viewed by others
References
J.C. Williams and E.A. Starke, Jr.: Progress in structural materials for aerospace systems. Acta Mater. 51, 5775–5799 (2003).
N.E. Prasad, A. Gokhale, and R. Wanhill: Aluminum-Lithium Alloys: Processing, Properties, and Applications (Butterworth-Heinemann, Oxford, England 2013).
M. Peters and C. Leyens: Aerospace and space materials. In Material Science and Engineering, Encyclopedia of Life Support Systems (EOLSS, Oxford, England).
J. Hirsch, B. Skrotzki, and G. Gottstein: Aluminium Alloys (Wiley, Weinheim, Germany, 2008).
E. Ghali, V.S. Sastri, and M. Elboujdaini: Corrosion Prevention and Protection: Practical Solutions (John Wiley & Sons, Chichester, United Kingdom, 2007).
K.P. Wong and R.C. Alkire: Local chemistry and growth of single corrosion pits in aluminum. J. Electrochem. Soc. 137, 3010–3015 (1990).
C-M. Liao and R.P. Wei: Galvanic coupling of model alloys to aluminum — A foundation for understanding particle-induced pitting in aluminum alloys. Electrochim. Acta 45, 881–888 (1999).
G. Chen, M. Gao, and R. Wei: Microconstituent-induced pitting corrosion in aluminum alloy 2024-T3. Corrosion 52, 8–15 (1996).
W.O. Soboyejo and T. Srivatsan: Advanced Structural Materials: Properties, Design Optimization, and Applications (CRC Press, Boca Raton, FL, 2006).
C. Giummarra, R. Rioja, G. Bray, P. Magnusen, and J. Moran: Al-Li alloys: Development of corrosion resistant, high toughness aluminium-lithium aerospace alloys. In Proceedings of the 11th International Conference on Aluminum Alloys (ICAA11), Aachen, Germany, 2008, pp. 176–188.
R.J. Rioja and J. Liu: The evolution of Al-Li base products for aerospace and space applications. Metall. Mater. Trans. A 43, 3325–3337 (2012).
W.R. Osório, L.C. Peixoto, D.J. Moutinho, L.G. Gomes, I.L. Ferreira, and A. Garcia: Corrosion resistance of directionally solidified Al-6Cu-1Si and Al-8Cu-3Si alloys castings. Mater. Des. 32, 3832–3837 (2011).
X. Zhang, Z.H. Jiang, Z.P. Yao, Y. Song, and Z.D. Wu: Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 51, 581–587 (2009).
B. Zaid, D. Saidi, A. Benzaid, and S. Hadji: Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 50, 1841–1847 (2008).
J.R. Davis: Corrosion of Aluminum and Aluminum Alloys (ASM International, Materials Park, OH, 1999).
W. Osório, J. Spinelli, I. Ferreira, and A. Garcia: The roles of macrosegregation and of dendritic array spacings on the electrochemical behavior of an Al-4.5 wt.% Cu alloy. Electrochim. Acta 52, 3265–3273 (2007).
W.R. Osorio, D.J. Moutinho, L.C. Peixoto, I.L. Ferreira, and A. Garcia: Macrosegregation and microstructure dendritic array affecting the electrochemical behaviour of ternary Al–Cu–Si alloys. Electrochim. Acta 56, 8412–8421 (2011).
W.R. Osorio, E.S. Freitas, J.E. Spinelli, M.V. Cante, C.R. Afonso, and A. Garcia: Assessment of electrochemical and mechanical behavior of hot-extruded powders and as-cast samples of Al-Ni alloys. Int. J. Electrochem. Sci. 7, 9946–9971 (2012).
G. Mearini and R. Hoffman: Tensile properties of aluminum/alumina multilayered thin films. J. Electron. Mater. 22, 623–629 (1993).
M. Saif, S. Zhang, A. Haque, and K. Hsia: Effect of native Al2O3 on the elastic response of nanoscale Al films. Acta Mater. 50, 2779–2786 (2002).
Z. Szklarska-Smialowska: Pitting corrosion of aluminum. Corros. Sci. 41, 1743–1767 (1999).
S. Wang and M. Starink: Precipitates and intermetallic phases in precipitation hardening Al–Cu–Mg–(Li) based alloys. Int. Mater. Rev. 50, 193–215 (2005).
B.C. Muddle and I. Polmear: The precipitate Ω phase in Al-Cu-Mg-Ag alloys. Acta Metall. 37, 777–789 (1989).
Z. Ahmad and B. Aleem: Effect of nano Al (Scx-1Zrx) precipitates on the mechanical and corrosion behavior of Al-2.5 Mg alloys. Mater. Corros. 62, 335–345 (2011).
K. Ralston, N. Birbilis, M. Weyland, and C. Hutchinson: The effect of precipitate size on the yield strength-pitting corrosion correlation in Al-Cu-Mg alloys. Acta Mater. 58, 5941–5948 (2010).
N. Birbilis, M. Cavanaugh, L. Kovarik, and R. Buchheit: Nanoscale dissolution phenomena in Al–Cu–Mg alloys. Electrochem. Commun. 10, 32–37 (2008).
K. Ralston, N. Birbilis, M. Cavanaugh, M. Weyland, B. Muddle, and R. Marceau: Role of nanostructure in pitting of Al-Cu-Mg alloys. Electrochim. Acta 55, 7834–7842 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, B., Li, CH., He, SC. et al. Corrosion behavior of 2099 Al-Li alloy in NaCl aqueous solution. Journal of Materials Research 29, 1344–1353 (2014). https://doi.org/10.1557/jmr.2014.121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2014.121