Abstract
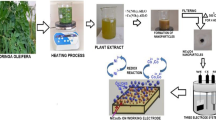
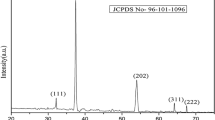
P-type NiO powders with an average crystallite size of 16 nm as shown by x-ray diffraction analysis were produced via biosynthesis using cactus plant extract. SEM showed that the NiO powders consisted of particles with sizes in the 20-35 nm range. A cyclic voltammetric study of the NiO nanopowders showed a quasi-reversible redox processes with the NiO powder showing potential for pseudo capacitance. Through these findings the use of natural Cactus extracts is hereby shown to be a cost-effective and environmentally friendly alternative for preparing Nickel oxide nanosized powders that can be of use in a variety of energy storage applications.
Similar content being viewed by others
References
Kuppusamy P., et al., Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications-An updated report. Saudi Pharmaceutical Journal, 2016. 24(4): p. 473–484.
Mondal S., et al., Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. Colloids and Surfaces B: Biointerfaces, 2011. 82(2): p. 497–504.
Siddique M.N., et al. Investigation of optical properties of nickel oxide nanostructures using photoluminescence and diffuse reflectance spectroscopy. in AIP Conference Proceedings. 2018. AIP Publishing.
Kelsall R.W., Hamley I.W., and Geoghegan M., Nanoscale science and technology. 2005: Wiley Online Library.
Bashir A., et al., Biosynthesis of NiO nanoparticles for photodegradation of free cyanide solutions under ultraviolet light. Journal of Physics and Chemistry of Solids, 2019.
Sun C., Li H., and Chen L., Nanostructured ceria-based materials: synthesis, properties, and applications. Energy & Environmental Science, 2012. 5(9): p. 8475–8505.
Ezhilarasi A.A., et al., Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. Journal of Photochemistry and Photobiology B: Biology, 2018. 180: p. 39–50.
Kaviyarasu K., et al., Synthesis and characterization studies of NiO nanorods for enhancing solar cell efficiency using photon upconversion materials. Ceramics International, 2016. 42(7): p. 8385–8394.
Gleiter H., Nanostructured materials: basic concepts and microstructure. Acta materialia, 2000. 48(1): p. 1–29.
Zhang F.-b., Zhou Y.-k., and Li H.-l., Nanocrystalline NiO as an electrode material for electrochemical capacitor. Materials Chemistry and Physics, 2004. 83(2-3): p. 260–264.
Bhatt M.D. and Lee J.Y., High capacity conversion anodes in Li-ion batteries: A review. International Journal of Hydrogen Energy, 2019. 44(21): p. 10852–10905.
Arico A.S., et al., Nanostructured materials for advanced energy conversion and storage devices, in Materials for sustainable energy: a collection of peer-reviewed research and review articles from Nature Publishing Group. 2011, World Scientific. p. 148–159.
Yang H., et al., Solid-state synthesis and electrochemical property of SnO2/NiO nanomaterials. Journal of alloys and compounds, 2008. 459(1-2): p. 98–102.
Fardood S.T., Ramazani A., and Moradi S., A novel green synthesis of nickel oxide nanoparticles using Arabic gum. Chemistry Journal of Moldova, 2017. 12(1): p. 115-8.
Beach E.R., et al., Solvothermal synthesis of crystalline nickel oxide nanoparticles. Materials Chemistry and Physics, 2009. 115(1): p. 371–377.
Anandan b. and Rajendran V., Morphological and size effects of NiO nanoparticles via solvothermal process and their optical properties. Materials Science in Semiconductor Processing, 2011. 14(1): p. 43–47.
Rahdar A., Aliahmad M., and Azizi Y., NiO nanoparticles: synthesis and characterization. 2015.
Xiang L., Deng X., and Jin Y., Experimental study on synthesis of NiO nano-particles. Scripta Materialia, 2002. 47(4): p. 219–224.
Maiti S., Pramanik A., and Mahanty S., Interconnected network of MnO2 nanowires with a “cocoonlike” morphology: redox couple-mediated performance enhancement in symmetric aqueous supercapacitor. ACS applied materials & interfaces, 2014. 6(13): p. 10754–10762.
Mayedwa N., et al., Green synthesis of nickel oxide, palladium and palladium oxide synthesized via Aspalathus linearis natural extracts: physical properties & mechanism of formation. Applied Surface Science, 2018. 446: p. 266–272.
Niu M., et al., Synthesis of nanoporous CuO/TiO2/Pd-NiO composite catalysts by chemical dealloying and their performance for methanol and ethanol electro-oxidation. Journal of Power Sources, 2017. 362: p. 10–19.
Ahmad T., et al., Magnetic and electrochemical properties of nickel oxide nanoparticles obtained by the reverse-micellar route. Solid State Sciences, 2006. 8(5): p. 425–430.
Nwanya A.C., et al., Electrochromic and electrochemical supercapacitive properties of room temperature PVP capped Ni (OH) 2/NiO thin films. Electrochimica Acta, 2015. 171: p. 128–141.
Zheng Y.-z., Ding H.-y., and Zhang M.-l., Preparation and electrochemical properties of nickel oxide as a supercapacitor electrode material. Materials Research Bulletin, 2009. 44(2): p. 403–407.
Zheng Y.-Z. and Zhang M.-L., Preparation and electrochemical properties of nickel oxide by molten-salt synthesis. Materials Letters, 2007. 61(18): p. 3967–3969.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gebretinsae, H., Welegergs, G., Matinise, N. et al. Electrochemical study of Nickel Oxide (NiO) nanoparticles from cactus plant extract. MRS Advances 5, 1095–1102 (2020). https://doi.org/10.1557/adv.2020.118
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2020.118