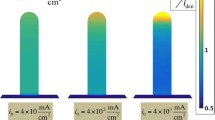
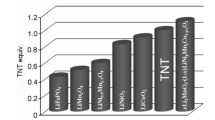
Abstract
The energy density of electrodeposition reactions makes them attractive for energy storage. Although its scientific inquiries nearly date back to the inception of electrochemistry, its behavior at microscopic dimensions (relevant to battery application) is mysteriously uncontrollable. We examine experimental reports of singular spatiotemporal evolutions with a hope to identify universality in deposition patterns. We conclude that a macroscopic mass transport instability cannot account for various growth morphologies and alludes to poorly understood materials interplay at smaller scales. We summarize representative characteristics of electrodeposition to encourage mechanistic investigations.
Similar content being viewed by others
References
Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J.-M. Li-O2 and Li-S Batteries with High Energy Storage. Nat. Mater. 2012, 11 (1), 19–29.
Bai, P.; Li, J.; Brushett, F. R.; Bazant, M. Z. Transition of Lithium Growth Mechanisms in Liquid Electrolytes. Energy Environ. Sci. 2016, 9 (10), 3221–3229. https://doi.org/10.1039/C6EE01674J.
Chen, K. H.; Wood, K. N.; Kazyak, E.; Lepage, W. S.; Davis, A. L.; Sanchez, A. J.; Dasgupta, N. P. Dead Lithium: Mass Transport Effects on Voltage, Capacity, and Failure of Lithium Met al Anodes. J. Mater. Chem. A 2017. https://doi.org/10.1039/c7ta00371d.
Mullins, W. W.; Sekerka, R. F. Stability of a Planar Interface during Solidification of a Dilute Binary Alloy. J. Appl. Phys. 1964. https://doi.org/10.1063/L1713333.
Sundström, L. G.; Bark, F. H. On Morphological Instability during Electrodeposition with a Stagnant Binary Electrolyte. Electrochim. Acta 1995. https://doi.org/10.1016/0013-4686(94)00379-F.
Khoo, E.; Zhao, H.; Bazant, M. Z. Linear Stability Analysis of Transient Electrodeposition in Charged Porous Media: Suppression of Dendritic Growth by Surface Conduction. J. Electrochem. Soc. 2019. https://doi.org/10.1149/2.1521910jes.
Lu, D.; Shao, Y.; Lozano, T.; Bennett, W. D.; Graff, G. L.; Polzin, B.; Zhang, J.; Engelhard, M. H.; Saenz, N. T.; Henderson, W. A. Failure Mechanism for Fast-charged Lithium Met al Batteries with Liquid Electrolytes. Adv. Energy Mater. 2015, 5 (3).
Shi, F.; Pei, A.; Vailionis, A.; Xie, J.; Liu, B.; Zhao, J.; Gong, Y.; Cui, Y. Strong Texturing of Lithium Met al in Batteries. Proc. Natl. Acad. Sci. U. S. A. 2017. https://doi.org/10.1073/pnas.1708224114.
Sun, F.; Osenberg, M.; Dong, K.; Zhou, D.; Hilger, A.; Jafta, C. J.; Risse, S.; Lu, Y.; Markötter, H.; Manke, I. Correlating Morphological Evolution of Li Electrodes with Degrading Electrochemical Performance of Li/LiCoO 2 and Li/S Battery Systems: Investigated by Synchrotron X-Ray Phase Contrast Tomography. ACS Energy Lett. 2018. https://doi.org/10.1021/acsenergylett.7b01254.
Mitchell, R. R.; Gallant, B. M.; Shao-Horn, Y.; Thompson, C. V. Mechanisms of Morphological Evolution of Li2O2 Particles during Electrochemical Growth. J. Phys. Chem. Lett. 2013. https://doi.org/10.1021/jz4003586.
Fan, F. Y.; Carter, W. C.; Chiang, Y. Mechanism and Kinetics of Li2S Precipitation in Lithium-Sulfur Batteries. Adv. Mater. 2015, 27 (35), 5203–5209.
Cheng, E. J.; Sharafi, A.; Sakamoto, J. Intergranular Li Met al Propagation through Polycrystalline Li6.25Al0.25La3Zr2O12 Ceramic Electrolyte. Electrochim. Acta 2017. https://doi.org/10.1016/j.electacta.2016.12.018.
Bieker, G.; Winter, M.; Bieker, P. Electrochemical in Situ Investigations of SEI and Dendrite Formation on the Lithium Met al Anode. Phys. Chem. Chem. Phys. 2015, 17 (14), 8670–8679.
Léger, C.; Elezgaray, J.; Argoul, F. Dynamical Characterization of One-Dimensional Stationary Growth Regimes in Diffusion-Limited Electrodeposition Processes. Phys. Rev. E — Stat. Physics, Plasmas, Fluids, Relat. Interdiscip. Top. 1998. https://doi.org/10.1103/PhysRevE.58.7700.
Monroe, C.; Newman, J. Dendrite Growth in Lithium/Polymer Systems a Propagation Model for Liquid Electrolytes under Galvanostatic Conditions. J. Electrochem. Soc. 2003, 150 (10), A1377–A1384.
Mistry, A.; Fear, C.; Carter, R.; Love, C. T.; Mukherjee, P. P. Electrolyte Confinement Alters Lithium Electrodeposition. ACS Energy Lett. 2019, 4 (1). https://doi.org/10.1021/acsenergylett.8b02003.
Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Met al. Nano Lett. 2017. https://doi.org/10.1021/acs.nanolett.6b04755.
Liu, W.; Lin, D.; Pei, A.; Cui, Y. Stabilizing Lithium Met al Anodes by Uniform Li-Ion Flux Distribution in Nanochannel Confinement. J. Am. Chem. Soc. 2016. https://doi.org/10.1021/jacs.6b08730.
Ding, F.; Xu, W.; Graff, G. L.; Zhang, J.; Sushko, M. L.; Chen, X.; Shao, Y.; Engelhard, M. H.; Nie, Z.; Xiao, J. Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism. J. Am. Chem. Soc. 2013, 135 (11), 4450–4456.
Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y. M.; Cui, Y. The Synergetic Effect of Lithium Polysulfide and Lithium Nitrate to Prevent Lithium Dendrite Growth. Nat. Commun. 2015. https://doi.org/10.1038/ncomms8436.
Fan, F. Y.; Chiang, Y.-M. Electrodeposition Kinetics in Li-S Batteries: Effects of Low Electrolyte/Sulfur Ratios and Deposition Surface Composition. J. Electrochem. Soc. 2017, 164 (4), A917–A922.
Viswanathan, V.; Thygesen, K. S.; Hummelshj, J. S.; Nrskov, J. K.; Girishkumar, G.; McCloskey, B. D.; Luntz, A. C. Electrical Conductivity in Li 2O 2 and Its Role in Determining Capacity Limitations in Non-Aqueous Li-O 2 Batteries. J. Chem. Phys. 2011. https://doi.org/10.1063/L3663385.
Aetukuri, N. B.; McCloskey, B. D.; Garciá, J. M.; Krupp, L. E.; Viswanathan, V.; Luntz, A. C. Solvating Additives Drive Solution-Mediated Electrochemistry and Enhance Toroid Growth in Non-Aqueous Li-O2 Batteries. Nat. Chem. 2015. https://doi.org/10.1038/nchem.2132.
Radin, M. D.; Monroe, C. W.; Siegel, D. J. Impact of Space-Charge Layers on Sudden Death in Li/O2 Batteries. J. Phys. Chem. Lett. 2015. https://doi.org/10.1021/acs.jpclett.5b01015.
Monroe, C.; Newman, J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152 (2), A396–A404.
Porz, L.; Swamy, T.; Sheldon, B. W.; Rettenwander, D.; Frömling, T.; Thaman, H. L.; Berendts, S.; Uecker, R.; Carter, W. C.; Chiang, Y. M. Mechanism of Lithium Met al Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017. https://doi.org/10.1002/aenm.201701003.
Lewis, J. A.; Cortes, F. J. Q.; Boebinger, M. G.; Tippens, J.; Marchese, T. S.; Kondekar, N.; Liu, X.; Chi, M.; McDowell, M. T. Interphase Morphology between a Solid-State Electrolyte and Lithium Controls Cell Failure. ACS Energy Lett. 2019. https://doi.org/10.1021/acsenergylett.9b00093.
Barai, P.; Higa, K.; Srinivasan, V. Lithium Dendrite Growth Mechanisms in Polymer Electrolytes and Prevention Strategies. Phys. Chem. Chem. Phys. 2017. https://doi.org/10.1039/c7cp03304d.
Maslyn, J. A.; Loo, W. S.; McEntush, K. D.; Oh, H. J.; Harry, K. J.; Parkinson, D. Y.; Balsara, N. P. Growth of Lithium Dendrites and Globules through a Solid Block Copolymer Electrolyte as a Function of Current Density. J. Phys. Chem. C 2018. https://doi.org/10.1021/acs.jpcc.8b06355.
Gribble, D. A.; Frenck, L.; Shah, D. B.; Maslyn, J. A.; Loo, W. S.; Mongcopa, K. I. S.; Pesko, D. M.; Balsara, N. P. Comparing Experimental Measurements of Limiting Current in Polymer Electrolytes with Theoretical Predictions. J. Electrochem. Soc. 2019. https://doi.org/10.1149/2.0391914jes.
Nagao, M.; Hayashi, A.; Tatsumisago, M. High-Capacity Li 2S-Nanocarbon Composite Electrode for All-Solid-State Rechargeable Lithium Batteries. J. Mater. Chem. 2012. https://doi.org/10.1039/c2jm16802b.
Barai, P.; Higa, K.; Ngo, A. T.; Curtiss, L. A.; Srinivasan, V. Mechanical Stress Induced Current Focusing and Fracture in Grain Boundaries. J. Electrochem. Soc. 2019. https://doi.org/10.1149/2.0321910jes.
Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J. O.; Hartmann, P.; Zeier, W. G.; Janek, J. Capacity Fade in Solid-State Batteries: Interphase Formation and Chemomechanical Processes in Nickel-Rich Layered Oxide Cathodes and Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2017. https://doi.org/10.1021/acs.chemmater.7b00931.
Gerber, L. C. H.; Frischmann, P. D.; Fan, F. Y.; Doris, S. E.; Qu, X.; Scheuermann, A. M.; Persson, K.; Chiang, Y. M.; Helms, B. A. Three-Dimensional Growth of Li2S in Lithium-Sulfur Batteries Promoted by a Redox Mediator. Nano Lett. 2016. https://doi.org/10.1021/acs.nanolett.5b04189.
Mistry, A. N.; Mukherjee, P. P. Electrolyte Transport Evolution Dynamics in Lithium-Sulfur Batteries. J. Phys. Chem. C 2018, 122 (32). https://doi.org/10.1021/acs.jpcc.8b05442.
Bergner, B. J.; Hofmann, C.; Schürmann, A.; Schröder, D.; Peppler, K.; Schreiner, P. R.; Janek, J. Understanding the Fundamentals of Redox Mediators in Li-O2 Batteries: A Case Study on Nitroxides. Phys. Chem. Chem. Phys. 2015. https://doi.org/10.1039/c5cp04505c.
Parker, J. F.; Chervin, C. N.; Pala, I. R.; Machler, M.; Burz, M. F.; Long, J. W.; Rolison, D. R. Rechargeable Nickel-3D Zinc Batteries: An Energy-Dense, Safer Alternative to Lithium-Ion. Science (80-.). 2017. https://doi.org/10.1126/science.aak9991.
Ta, K.; See, K. A.; Gewirth, A. A. Elucidating Zn and Mg Electrodeposition Mechanisms in Nonaqueous Electrolytes for Next-Generation Met al Batteries. J. Phys. Chem. C 2018. https://doi.org/10.1021/acs.jpcc.8b00835.
Wang, D.; Gao, X.; Chen, Y.; Jin, L.; Kuss, C.; Bruce, P. G. Plating and Stripping Calcium in an Organic Electrolyte. Nat. Mater. 2017, 17, 16.
Ta, K.; Zhang, R.; Shin, M.; Rooney, R. T.; Neumann, E. K.; Gewirth, A. A. Understanding Ca Electrodeposition and Speciation Processes in Nonaqueous Electrolytes for Next-Generation Ca-Ion Batteries. ACS Appl. Mater. Interfaces 2019. https://doi.org/10.1021/acsami.9b04926.
Rajput, N. N.; Qu, X.; Sa, N.; Burrell, A. K.; Persson, K. A. The Coupling between Stability and Ion Pair Formation in Magnesium Electrolytes from First-Principles Quantum Mechanics and Classical Molecular Dynamics. J. Am. Chem. Soc. 2015. https://doi.org/10.1021/jacs.5b01004.
Samuel, D.; Steinhauser, C.; Smith, J. G.; Kaufman, A.; Radin, M. D.; Naruse, J.; Hiramatsu, H.; Siegel, D. J. Ion Pairing and Diffusion in Magnesium Electrolytes Based on Magnesium Borohydride. ACS Appl. Mater. Interfaces 2017. https://doi.org/10.1021/acsami.7b15547.
Author information
Authors and Affiliations
Corresponding author
Additional information
ORCID: 0000-0002-4359-4975
ORCID: 0000-0002-1248-5952
Rights and permissions
About this article
Cite this article
Mistry, A., Srinivasan, V. On our Limited Understanding of Electrodeposition. MRS Advances 4, 2843–2861 (2019). https://doi.org/10.1557/adv.2019.443
Published:
Issue Date:
DOI: https://doi.org/10.1557/adv.2019.443