Abstract
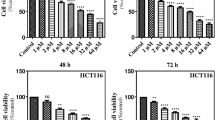
L-Asparaginase is a potential therapeutic agent owing to its anti-tumor activity. We have previously characterized a thermostable L-asparaginase (TK1656F from Thermococcus kodakaraensis that exhibits highest ever reported enzymatic activity, no glutaminase activity and high stability. In this study we have compared the cytotoxicity and anti-proliferative activity of TK1656 and a commercially available L-asparaginase from Escherichia coli on cancerous cells. Despite of the thermophilic origin, the amount of TK1656 required to inhibit equivalent growth of HeLa cells at 37°C was three times less than that of the L-asparaginase from E. coli. It is worth mentioning that TK1656 showed much reduced cytotoxic effect on non-cancerous cells as compared to the E. coli counterpart. Furthermore, TK1656 was found to potentiate induction of apoptosis of cancerous cells. Overall, our findings suggest that TK1656 harbors a remarkable potential for the cancer chemotherapeutics.
Similar content being viewed by others
References
Abakumova O.Y., Podobed O.V., Karalkin P.A., Kondakova L.I. & Sokolov N.N. 2013. Antitumor activity of L-asparaginase from Erwinia carotovora against different human and animal leukemic and solid tumor cell lines. Biochemistry (Moscow) 6: 307–316.
Anese M., Quarta B., Peloux L. & Calligaris S. 2011. Effect of formulation on the capacity of L-asparaginase to minimize acrylamide formation in short dough biscuits. Food Res. Int. 44: 2837–2842.
Arshad M.N., Nisar M.A., Khurshid M., Hussain S.Z., Maqsood U., Asghar M.T. & Nazir J. 2015. Molecular basis of arsenite (As+3)-induced acute cytotoxicity in human cervical epithelial carcinoma cells. Libyan J. Med. 10: 26875.
Broome J.D. 1963. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J. Exp. Med. 118: 99–120.
Chohan S.M. & Rashid N. 2013. TK1656, a thermostable L-asparaginase from Thermococcus kodakaraensis, exhibiting highest ever reported enzyme activity. J. Biosci. Bioeng. 116: 438–443.
Hendriksen H.V., Kornbrust B.A., Astergaard P.R. & Stringer M.A. 2009. Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from Aspergillus oryzae. J. Agric. Food Chem. 57: 4168–4176.
Huang L., Liu Y., Sun Y., Yan Q. & Jiang Z. 2014. Biochemical characterization of a novel L-asparaginase with low glutami-nase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl. Environ. Microbiol. 80: 1561–1569.
Khan A.A., Khurshid M., Khan S. & Alshamsan A. 2013. Gut microbiota and probiotics: current status and their role in cancer therapeutics. Drug Develop. Res. 74: 365–375.
Khan A.A, Shrivastava A. & Khurshid M. 2012. Normal to cancer microbiome transformation and its implication in cancer diagnosis. Biochim. Biophys. Acta 1826: 331–337.
Khurshid M., Aslam B., Nisar M.A., Akbar R., Rahman H., Khan A.A. & Rasool M.H. 2015. Bacterial munch for infants: potential pediatric therapeutic interventions of probiotics. Future Microbiol. 10: 1881–1895.
Narta U.K., Kanwar S.S. & Azmi W. 2007. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit. Rev. Oncol. Hematol. 61: 208–221.
Oshikane H., Sheppard K., Fukai S., Nakamura Y., Ishitani R., Numata T., Sherrer R.L., Feng L., Schmitt E., Panvert M., Blanquest S., Mechulam Y., Söll D. & Nureki O. 2006. Structural basis of RNA-dependent recruitment of glutamine to the genetic code. Science 312: 1950–1954.
Pritsa A.A., Papazisis K.T., Kortsaris A.H., Geromichalos G.D. & Kyriakidis D.A. 2001. Antitumor activity of L-asparaginase from Thermus thermophilus. Anticancer Drugs 12: 137–142.
Raish M., Khurshid M., Ansari M.A., Chaturvedi P.K., Bae S.M., Kim J.H., Park E.K., Park D.C. & Ahn W.S. 2012. Analysis of molecular cytogenetic alterations in uterine leiomyosarcoma by array-based comparative genomic hybridization. J. Cancer Res. Clin. Oncol. 138: 1173–1186.
Ramya L.N., Doble M., Rekha V.P. & Pulicherla K.K. 2012. L-Asparaginase as potent anti-leukemic agent and its significance of having reduced glutaminase side activity for better treatment of acute lymphoblastic leukaemia. Appl. Biochem. Biotechnol. 167: 2144–2159.
Schwartz R.S. 1969. Immunosuppression by L-asparaginase. Nature 224: 275–276.
Shrivastava A., Khan A.A., Khurshid M., Kalam M.A., Jain S.K. & Singhai P.K. 2016. Recent developments in L-asparaginase discovery and its potential as anticancer agent. Crit. Rev. Oncol. Hematol. 100: 1–10.
Waring P. & Mullbacher A. 1999. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 77: 312–317.
Yun M.K., Nourse A., White S.W., Rock C.O. & Heath R.J. 2007. Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli L-asparaginase I. J. Mol. Biol. 369: 794–811.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chohan, S.M., Nisar, M.A., Rashid, N. et al. TK1656, an L-asparaginase from Thermococcus kodakarensis, a novel candidate for therapeutic applications. Biologia 71, 1315–1319 (2016). https://doi.org/10.1515/biolog-2016-0168
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2016-0168