Abstract
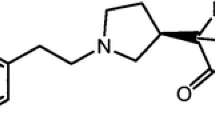
The capillary zone electrophoresis method was developed for the chiral separation of R,S-cinacalcet. Cyclodextrins with different substituents were tested in both acidic and alkaline background electrolytes. The non-ionic cyclodextrin, 2-hydroxypropyl-γ-cyclodextrin, was selected as the best chiral selector. The separation was performed using a positive voltage in a phosphate buffer at pH 2.5. The analytes studied were separated within 12 min. The proposed method was applied to the analysis of tablets containing R-cinalcalcet as the active substance. The enantiopurity of R-cinacalcet in the tablets studied was confirmed. Subsequently, the analysis of tablets spiked with S-cinacalcet (chiral impurity) was also performed. The method here presented makes possible the determination of 0.1 % of S-cinacalcet in tablets. The analytical characteristics of the method, such as linearity, recovery and RSD values of the peak area and the migration time, were evaluated. The inter-day RSD values of the peak area and the migration time were lower than 3.71 % and 1.3 %, respectively.
Similar content being viewed by others
References
Ahuja, S. (2007). Assuring quality of drugs by monitoring impurities. Advanced Drug Delivery Reviews, 59, 3–11. DOI: 10.1016/j.addr.2006.10.003.
Amin, N. C., Blanchin, M. D., Aké, M., Fabre, H. (2012). Capillary electrophoresis methods for the analysis of an-timalarials. Part I. Chiral separation methods. Journal of Chromatography A, 1264, 1–12. DOI: 10.1016/j.chroma.2012. 09.057.
Bhushan, R., & Dubey, R. (2011). Indirect reversed-phase high-performance liquid chromatographic and direct thin-layer chromatographic enantioresolution of (R,S)-cinacalcet. Biomedical Chromatography, 25, 674–679. DOI: 10.1002/ bmc.1502.
Blanco, M., Valverde, I. (2003). Choice of chiral selector for enantioseparation by capillary electrophoresis. TrAC Trends in Analytical Chemistry, 22, 428–439. DOI: 10.1016/s0165-9936(03)00705-2.
Blaschke, G., Chankvetadze, B. (2000). Enantiomer separation of drugs by capillary electromigration techniques. Journal of Chromatography A, 875, 3–25. DOI: 10.1016/s0021-9673(00)00134-5.
Caner, H., Groner, E., Levy, L., Agranat, I. (2004). Trends in the development of chiral drugs. Drug Discovery Today, 9, 105–110. DOI: 10.1016/s1359-6446(03)02904-0.
Chankvetadze, B. (2007). Enantioseparations by using capillary electrophoretic techniques: The story of 20 and a few more years. Journal of Chromatography A, 1168, 45–70. DOI: 10.1016/j.chroma.2007.08.008.
Choulwar, A. K., Mungantiwar, A. A., Chintamaneni, M. (2011). A comparative, bioequivalence study to evaluate the safety and pharmacokinetic profile of single dose cinacalcet hydrochloride tablets in healthy, adult, human subjects under fed conditions. Annals of Biological Research, 2, 35–50.
De Klerck, K., Mangelings, D., Vander Heyden, Y. (2012). Supercritical fluid chromatography for the enantioseparation of pharmaceuticals. Journal of Pharmaceutical and Biomedical Analysis, 69, 77–92. DOI: 10.1016/j.jpba.2012.01.021.
Douša, M., Brichác, J. (2012). Chiral chromatography studies of chemical behavior of cinacalcet on polysaccharide chiral reversed-phase HPLC stationary phases. Journal of AOAC International, 95, 1639–1643. DOI: 10.5740/jaoacint.11-351.
Escuder-Gilabert, L., Martín-Biosca, Y., Medina-Hernández, M. J., Sagrado, S. (2014). Cyclodextrins in capillary elec-trophoresis: Recent developments and new trends. Journal of Chromatography A, 1357, 2–23. DOI: 10.1016/j.chroma.2014. 05.074.
FDA (2008). Guidance for industry Q3A impurities in new drug substances, Revision 2. Silver Spring, MD, USA: Food and Drug Administration.
ICH (2005). ICH harmonised tripartite guideline: Validation of analytical procedures: Text and methodology Q2(R1), Current Step 4 version (incorporated in November 2005). Geneva, Switzerland: ICH.
ICH (2006). ICH harmonised tripartite guideline: Impurities in new drug products Q3B(R2), Current Step 4 version (dated 2 June 2006). Geneva, Switzerland: ICH.
Jouyban, A., Kenndler, E. (2008). Impurity analysis of pharmaceuticals using capillary electromigration methods. Elec-trophoresis, 29, 3531–3551. DOI: 10.1002/elps.200800054.
Kalíková, K., Riesová, M., Tesarová, E. (2012). Recent chiral selectors for separation in HPLC and CE. Central European Journal of Chemistry, 10, 450–471. DOI: 10.2478/s11532-011-0142-3.
Mathad, V. T., Niphade, N. C., Shinde, G. B., Ippar, S. S., Deshmukh, S. P., Panchangam, R. K. (2011). U.S. Patent Application No. US20110319663. Alexandria, VA, USA: U.S. Patent and Trademark Office.
Nemeth, E. F., Heaton, W. H., Miller, M., Fox, J., Balandrin, M. F., Van Wagenen, B. C., Colloton, M., Karbon, W., Scherrer, J., Shatzen, E., Rishton, G., Scully, S., Qi, M., Harris, R., Lacey, D., Martin, D. (2004). Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. The Journal of Pharmacology and Experimental Therapeutics, 308, 627–635. DOI: 10.1124/jpet.103.057273.
Plotka, J. M., Biziuk, M., Morrison, C, Namiesnik, J. (2014). Pharmaceutical and forensic drug applications of chiral supercritical fluid chromatography. TrAC Trends in Analytical Chemistry, 56, 74–89. DOI: 10.1016/j.trac.2013.12.012.
Ramisetti, N. R., Bompelli, S. (2014). LC-MS/MS determination of cinacalcet enantiomers in rat plasma on Chirobi-otic V column in polar ionic mode: application to a pharma-cokinetic study. Biomedical Chromatography, 28, 1846–1853. DOI: 10.1002/bmc.3229.
Ravinder, V., Ashok, S., Varma, M. S., Babu, C. V. R., Shanker, K., Balaswamy, G. (2009). A validated chiral LC method for the enantiomeric separation of Cinacalcet hydrochloride. Chromatographia, 70, 229–232. DOI: 10.1365/s10337-009-1129-5.
Rezanka, P., Navrátilová, K., Rezanka, M., Král, V., Sýkora, D. (2014). Application of cyclodextrins in chiral capillary electrophoresis. Electrophoresis, 35, 2701–2721. DOI: 10.1002/elps.201400145.
Roy, J. (2002). Pharmaceutical impurities A mini-review. AAPS PharmSciTech, 3, article 6. DOI: 10.1208/pt030206.
Scriba, G. K. E. (2002). Selected fundamental aspects of chiral electromigration techniques and their application to pharmaceutical and biomedical analysis. Journal of Pharmaceutical and Biomedical Analysis, 27, 373–399. DOI: 10.1016/s0731-7085(01)00653-7.
Shahapuni, I., Monge, M., Oprisiu, R., Mazouz, H., Westeel, P. F., Morinière, P., Massy, Z., Choukroun, G., Fournier, A. (2006). Drug insight: renal indications of calcimimetics. Nature Reviews Nephrology, 2, 316–325. DOI: 10.1038/ncp-neph0191.
Tsioupi, D. A., Stefanvan Staden, R. I., Kapnissi-Christo-doulou, C. P. (2013). Chiral selectors in CE: Recent developments and applications. Electrophoresis, 34, 178–204. DOI: 10.1002/elps.201200239.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ginterová, P., Znaleziona, J., Knob, R. et al. Enantiomeric purity control of R-cinacalcet in pharmaceutical product by capillary electrophoresis. Chem. Pap. 70, 1024–1030 (2016). https://doi.org/10.1515/chempap-2016-0047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2016-0047