Abstract
Background
Alzheimer’s disease (AD) is the most common neurodegenerative disease worldwide, and an updated quantification of its impact on morbidity, disability, and mortality is warranted. We conducted a systematic literature review, focusing on the past decade, to characterize AD and assess its impact on affected individuals.
Methods
Searches of Embase, MEDLINE, and the Cochrane Library were conducted on August 7, 2020 and updated on November 10, 2021. Observational studies from any country reporting incidence, prevalence, comorbidities, and/or outcomes related to disability and mortality/life expectancy, in people with mild cognitive impairment (MCI) due to AD, or mild, moderate, or severe AD dementia, were considered relevant.
Results
Data were extracted from 88 studies (46 incidence/prevalence; 44 comorbidities; 25 mortality-/disability-related outcomes), mostly from Europe, the USA, and Asia. AD dementia diagnosis was confirmed using biomarkers in only 6 studies. Estimated 5-year mortality in AD was 35%, and comorbidity prevalence estimates varied widely (hypertension: 30.2–73.9%; diabetes: 6.0–24.3%; stroke: 2.7–13.7%). Overall, people with AD dementia were more likely to have cardiovascular disease or diabetes than controls, and 5-year mortality in people with AD dementia was double that in the age- and year-matched general population (115.0 vs 60.6 per 1,000 person-years).
Conclusions
AD is associated with excess morbidity and mortality. Future longitudinal studies of population aging, incorporating biomarker assessment to confirm AD diagnoses, are needed to better characterize the course of MCI due to AD and AD dementia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia affects an estimated 57 million people worldwide (1), and Alzheimer’s disease (AD) is the most frequent cause of dementia (2). AD dementia is characterized by specific changes in the brain, notably the deposition of beta-amyloid protein as extracellular plaques and the presence of neurofibrillary tangles composed of phosphorylated tau protein (3). AD also has a preclinical phase and prodromal manifestations that are usually not taken into account in prevalence estimates.
Deficits in memory, language, and problem-solving may initially present as mild cognitive impairment (MCI) (4), which is not severe enough to affect functioning, whereas dementia is characterized by progressive cognitive deterioration and limitations to functioning (5) and is ultimately fatal. Nearly all individuals with MCI due to AD progress eventually to AD dementia if observed for long enough (6); in a cohort of 18,000 US individuals, the annual probability of progression to mild AD dementia at the age of 65 years for those with MCI due to AD was estimated to be 21% (7). Research diagnostic criteria for MCI due to AD and AD dementia incorporate assessment of biomarkers, alongside clinical evaluations and neuropsychological testing (8); these guidelines do not currently recommend the use of biomarkers to diagnose AD in clinical practice (6, 9). Although the vast majority of treated patients with AD receive therapies only for symptoms, two treatments have been approved by the US Food and Drug Administration for MCI due to AD and mild AD dementia (10, 11). As these therapies target beta-amyloid (10, 11), an increase in biomarker-defined AD is anticipated for the future.
The total number of deaths attributable to AD dementia has increased during the past 20 years, making it one of the leading causes of death in the USA (12). AD dementia is usually diagnosed in people in their mid-sixties or older; consequently, people with AD dementia commonly have comorbidities, such as cardiovascular disease (CVD). There is evidence that risk factors common to comorbidities and AD, such as chronic inflammation, can mean that individuals with comorbidities are at an increased risk of developing AD (13); however, the interplay between comorbidities, development and progression of AD dementia, and patient outcomes remains incompletely understood (14). Many large burden of disease studies, such as the Global Burden of Disease studies (15), group AD dementia with other dementias under the overarching term ‘dementia’, and as such, there is little information available to assess the relationship between comorbidity burden, disability, and mortality in individuals with AD specifically.
AD remains a key public health priority worldwide, and identification and synthesis of recent data are required to quantify its true impact. We designed and conducted a systematic literature review (SLR) to identify current evidence on the prevalence and incidence of AD and its effects on mortality and life expectancy, and to assess the relationships between AD, comorbidities, and disability.
Materials and Methods
Systematic literature review
The SLR was designed to identify relevant data on the burden of AD from observational studies of people with MCI due to AD, or mild, moderate, or severe AD dementia. Outcomes of interest were incidence and prevalence, comorbidities, mortality, life expectancy, and disability. To identify evidence on the latter two outcomes, search terms were included for years of life lost (YLLs), a measure of premature mortality, years lived with disability (YLDs), and disability-adjusted life-years (DALYs), which express the impact of disability in terms of how many years of healthy life have been lost. The study protocol was designed and conducted in line with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16), and registered with PROSPERO (registration number: CRD42022297125).
Searches were conducted on August 7, 2020, and updated on November 10, 2021 to capture literature published since the original searches; Table S1 and Table S2 show the search strings. Titles and abstracts were screened by one researcher to determine whether they met the eligibility criteria (Table 1). For inclusion, studies were required to specify which severity or stages of AD were represented in the population; all staging methods and criteria were considered valid. Journal articles published from 2010 onwards and conference abstracts published from 2015 onwards were considered relevant in screening. Primary publications were included, but review articles were not; reference lists from systematic reviews and meta-analyses were cross-checked for relevant articles. Only English language publications were included. There was no restriction by study geography. All publications meeting the criteria were obtained as full articles and reassessed against the eligibility criteria.
Data extraction and prioritization
Data were extracted from studies published 2015–present, to identify the most recent and relevant data. For prevalence and incidence, data from studies that selected populations for the presence of dementia or neurological conditions were not extracted, because those data would not provide an accurate estimate of AD prevalence or incidence in the general population. For comorbidities, data were extracted from publications that also reported epidemiology or mortality data and, to assess the potential impact of AD on comorbidity prevalence, from any publications that reported comorbidities for both individuals with AD and controls. Detailed data, including study setting and methods, patient characteristics, and study results, were entered into a data extraction table and quality checked by an independent reviewer.
In this manuscript, only AD-specific data are reported, and data from populations that included individuals with non-AD dementias, only some of whom had AD, were not considered relevant. It should be noted, however, that because most AD diagnoses were not confirmed using biomarkers, some people diagnosed with AD dementia in the included studies are likely to have other types of dementia, or mixed dementia.
In this manuscript, we have used data from relevant studies to address two key aims. First, we characterize AD, collating data on incidence and prevalence, comorbidities in people with AD, mortality, and survival. Second, we aimed to understand the impact of AD on comorbidities and mortality by assessing studies comparing data between individuals with and without AD. One study that assessed the disability associated with AD as part of the wider impact of dementia is also discussed in the manuscript.
Results
Search results
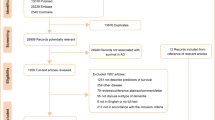
In total, 6,259 papers identified in the original SLR and 1,415 papers identified in the SLR update were included for screening by abstract and title, resulting in 540 references included for full-paper review (see PRISMA flow diagram in Figure 1). Cross-checks with a separate targeted literature review on AD resulted in the inclusion of three additional references, and three references were included from congress searches.
PRISMA diagram for the SLR
The two sets of numbers at the search and screening stages indicate the numbers of references identified in the original SLR conducted in August 2020 and the update in November 2021, respectively. From full-paper review onwards, duplicates across the two searches had been identified and removed. AD, Alzheimer’s disease; RWE, real-world evidence; SLR, systematic literature review.
In total, 258 references met the inclusion criteria for full-paper review, and data were extracted from 88 studies. Some studies reported more than one type of data; Figure S1A shows the overlap between categories. Overall, 46 studies reported epidemiology data (17–62), 44 studies reported data on comorbidities (17, 19, 25, 27, 28, 34, 36, 37, 39, 42, 44, 60, 63–94), and 25 studies reported data on mortality, survival, and disability (30, 36, 42, 44, 60, 81, 82, 84, 85, 87, 88, 90–92, 94–104).
Data sources and study designs
In total, 36 studies reported data from Europe (20, 22–24, 28, 30, 33, 35, 37, 48, 52, 55–58, 60, 63, 65, 73–77, 82, 86–88, 90–93, 95, 96, 98, 101, 104) and 22 studies reported data from Asia (25, 31, 38, 39, 43, 59, 62, 66, 68–72, 78–81, 83, 85, 89, 94, 99). Seventeen studies reported data from the USA (17–19, 26, 34, 36, 40–42, 45, 49–51, 61, 100, 102, 103). Two studies reported data from each of Australia (27, 44), North Africa (32, 46), Brazil (54, 64), and one study reported data from Cuba (67). Four studies reported data from multiple countries (47, 53, 84, 97), and two did not report country (21, 29). The countries represented in the data identified are shown in Figure S1B. Approximately half of the studies identified used primary data collection and a similar number were retrospective studies using secondary data. Most data were from dementia-based cohorts, national registries, and electronic medical records; however, in some publications individuals were recruited directly from hospitals or primary care settings.
AD diagnosis and staging
In most studies, AD dementia was diagnosed using neurological and neuropsychiatric assessments, and in some cases brain imaging and laboratory tests. Most frequently, diagnoses were made using the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria, the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria, or the criteria proposed by the National Institute on Aging–Alzheimer’s Association (NIA-AA) workgroup. A minority of publications reported any AD biomarker data for included individuals and only six studies used biomarkers to confirm the diagnosis of AD dementia (23, 30, 33, 38, 73, 92). Four of these studies assessed both amyloid and tau levels in cerebrospinal fluid (CSF) (total tau and p-tau in three (30, 73, 92); p-tau in one (33)), of which one also conducted brain magnetic resonance imaging (MRI) (92). Honda et al. (2019) reported the results of an autopsy series assessing tau pathology over 31 years in Japan (38), and the remaining publication (Bouteloup et al., 2019) carried out MRI and amyloid positron emission tomography (PET) scans on a sub-sample of individuals, but did not assess tau or p-tau levels (23).
Characterization of AD
Prevalence of MCI due to AD and AD dementia
Estimates of AD prevalence varied widely across studies, owing to differences in study settings and the ages of the populations being assessed, among other factors (Table S2). Six studies reported single estimates of AD prevalence in the general older population or in population-based cohorts (32, 37, 39, 49, 52, 56). Only one of these studies (Vlachos et al., 2020) reported the prevalence of MCI due to AD, which was found to be 8.4% in a cohort of older (≥ 65 years) people in Greece (56). The prevalence of AD dementia was reported in four studies (32, 37, 39, 49), and ranged from 0.8% in Italy (Grande et al., 2020) (37) to 14.5% in the USA (Rajan et al., 2019) (49). The sixth study (Ruano et al., 2019) reported the combined prevalence of MCI due to AD, and AD dementia, in Portugal, which together was 1.8% (1.4% MCI due to AD and 0.4% AD) (52). Three studies reported changes in AD prevalence over time; however, the settings, study periods and patient characteristics, such as age, in these analyses varied (Table S2).
Incidence of MCI due to AD and AD dementia
Six studies reported estimates of AD incidence in the general population (Table S2) (20, 40, 49, 57, 58, 62). Kirson et al. (2020) estimated the incidence of AD dementia in a random sample of US Medicare beneficiaries using International Classification of Diseases codes. Incidence decreased from 1.5% in 2007 to 1.1% in 2014 (40). Rajan et al. (2019) reported an incidence of 2.3%, based upon data from individuals ≥ 65 years old in the Chicago Health and Aging Project, a population-based study standardized to the US population (49). Yuan et al. (2016) reported that the crude incidence of AD dementia per 1,000 person-years was 4.9 in 16,921 individuals aged 55 years or older in China (62), and Andreu-Reinon et al. (2020) reported an age-adjusted incidence of AD dementia of 5.5 per 1,000 person-years among people ≥ 65 years old in a Spanish population, standardized to the 2013 European Standard Population (20). The highest estimates were reported in two analyses from the Hellenic Longitudinal Investigation of Aging and Diet study in Greece, which included individuals aged 65 years old or over. The incidence of MCI due to AD per 1,000 person-years was 34.1 (Vlachos et al., 2021a) (57), and the incidence of AD dementia was 16.3 (Vlachos et al., 2021b) (58).
AD dementia prevalence by stage
Four studies reported estimates of the prevalence of AD dementia by stage at diagnosis or referral (Table 2); only one included MCI in these assessments (Yuan et al., 2021) (61). Two were cross-sectional studies of individuals referred to outpatient clinics. Souza et al. (2019) found that, in a cohort of 256 individuals in Brazil, most presented with mild AD dementia (54). In the second study, El Tallawy et al. (2019) reported data from 126 people in Egypt invited for an assessment due to suspected dementia. Similar proportions had mild and moderate AD dementia, and the remainder had severe AD dementia (32). An analysis by Yuan et al. (2021) of individuals in the population-based US Framingham Heart Study found that mild AD dementia was the most prevalent stage of AD dementia at diagnosis (50.4% of 607 individuals with AD dementia). In the full population (N = 1192), which included individuals with MCI and AD dementia, 49.1% had MCI (29.5% MCI that did not progress to AD; 19.5% MCI that progressed to AD), 25.7% had mild AD dementia, 15.4% had moderate AD dementia, and 9.8% had severe AD dementia. (61). In a study by Chua et al. (2019) assessing data from people who presented with AD dementia at a geriatric medicine memory clinic in Singapore between 2005 and 2017 (25), the proportion presenting with mild AD dementia increased whereas the proportion presenting with moderate AD dementia decreased over time (Figure S2).
Prevalence of comorbidities in AD
Thirteen studies (Figure 2A and Table S4) reported data on hypertension, diabetes (type 1 or 2), or CVD in patient cohorts with MCI due to AD or AD dementia, including one study (Xu et al., 2021) (60) that comprised people with AD dementia or mixed AD dementia (28, 36, 37, 60, 80, 81, 84, 88–90, 92–94). These data were typically included in summary tables of baseline characteristics, and the development of comorbidities relative to the timing of AD dementia diagnosis was not clear in most publications. The prevalence of hypertension ranged between 30.2% (Yeh et al., 2020, in people with early-onset AD) (94) and 73.8%/73.9% (Xu et al., 2021, in acetylcholinesterase inhibitor users and non-users, respectively) (60). The prevalence of diabetes ranged between 6.0% (Staekenborg et al., 2016, in people with AD dementia [diagnosis supported by biomarker assessment] with rapid mortality) (92) and 24.3% (Gracner et al., 2021, type 2 diabetes only) (36). The prevalence of stroke ranged between 2.7% (Grande et al., 2020) (37) and 13.7% (Yeh et al., 2020, in people with late-onset AD) (94). Data on the prevalence of various other cardiovascular conditions and risk factors were also reported, including, heart failure, myocardial infarction, and dyslipidemia (Table S4).
Prevalence of selected comorbidities in people with AD (A), and prevalence of hypertension (B), diabetes (C) and stroke (D) in people with AD and control individuals
Further details: Asterisk (*) denotes studies that specify T2D. For panel (A), studies are presented in order of increasing prevalence. For panel (B), studies are presented by region. Panel (A): Chen et al. 2019: study period 2012–2016. ‘Alive’ refers to individuals newly diagnosed with probable AD who were alive at the end of the study period (age [mean ± SD], alive: 85.8 ± 3.1 years; deceased: 87.9 ± 4.7 years) (81). Chen et al. 2020: study period 2015–2016 (80). de Mauleon et al 2017: study period 2007–2011; ‘stroke’ is reported as history of or ongoing ischemic and hemorrhagic stroke (28). Gracner et al. 2021: study period 2011–2013 (36). Grande et al. 2020: study period 2002–2006; hypertension, diabetes and stroke are reported for the ≥ 10-year medical history (37). Kelaiditi et al. 2016: study period 2003–2005; the 12 European countries are not specified (84). Pan et al. 2021: study period 2001–2019 (89). Rhodius-Meester et al. 2018: study period 2000–2014; alive refers to individuals alive after mean ± SD follow-up of 4.9 ± 2.0 years; hypertension and diabetes are reported as history of hypertension and/or use of antihypertensive drugs, and history of diabetes mellitus and/or use of antidiabetic drugs, respectively (90). Staekenborg et al. 2016: study period 2000–2013; rapid mortality is defined as death within ≤ 2 years after diagnosis; stroke includes TIA (92). The prevalence of hypertension is reported as 38% in the publication, but the number of cases is reported as 20 of 55 individuals. Vu et al. 2020: study period 2005–2011; comorbidities are reported as prevalence at the time of AD diagnosis (93). Xu et al. 2021: study period 2007–2017 (60). Yeh et al. 2020: Study period 2000–2012. mean ± SD age for early-onset AD group: 61 ± 4; late-onset AD group: 78 ± 6 (94). Panels (B–D): Andersen et al. 2011: study period 2006–2008 (63). de Lima et al. 2020: study period NR (64). Grande et al. 2020: study period 2002–2006 (37). Ilmaniemi et al. 2019: study period 2005–2015 (65). Kuyumcu et al. 2012: study period NR (66). Lanyau-Dominguez et al. 2020: study period NR (67). Lee et al. 2017: study period 2000–2010 (69). Lyou et al. 2018: study period 2004–2013 (72). Tolppanen et al. 2013a: study period 2002–2009; ‘stroke’ is reported as history of stroke (74). Tolppanen et al. 2016: study period 2005–2012 (77). Vu et al. 2020: study period 2005–2011 (93). Wang et al. 2018: study period 2001–2011 (78). AChEI, acetylcholinesterase inhibitor; AD, Alzheimer’s disease; NR, not reported; OR, odds ratio; RWE, real-world evidence; SLR, systematic literature review; T2D, type 2 diabetes; TIA, transient ischemic attack.
Mortality in AD dementia
Six studies reported estimates of all-cause mortality in AD dementia (Table 3) (30, 44, 90, 94, 98, 104). Two additional studies reported data on life expectancy only (44, 81) (not presented). Mortality estimates could not be readily compared across all studies owing to differences in follow-up periods; however, three studies had a similar duration of follow-up. Mank et al. (2021) used data from the Amsterdam Dementia Cohort (98), and Rhodius-Meester et al. (2018) conducted a prospective study using the same cohort (90), meaning that the populations in the two studies were similar in terms of age (Mank et al., 2021: mean ± standard deviation [SD]: 65 ± 7 years; Rhodius-Meester et al., 2018: 66 ± 7 years [alive at end of follow-up] and 69 ± 9 years [deceased at end of follow-up]); all had AD dementia. Degerman Gunnarsson et al. (2016) used data from a memory clinic in Sweden. Included individuals had a median age of 70 years (range: 46–86) and 57% had MCI due to AD rather than AD dementia (30); AD diagnosis was supported by assessment of CSF total tau levels. In each of these three analyses, 35% of people died over approximately 5 years of follow-up.
Burden of AD
Comorbidities
Nineteen studies compared the prevalence of comorbidities in people with AD dementia with those in the general population or in cognitively healthy control individuals (37, 63–79, 93) (Table S5).
Nine studies reported data on the prevalence of hypertension in people with AD dementia, compared with control individuals (Figure 2B) (37, 63, 64, 66, 67, 69, 72, 78, 93). The prevalence of hypertension varied across studies but was generally similar in people with AD dementia and controls. Lyou et al. (2018) (72) found a higher prevalence in the cohort with AD dementia (71.7% vs 55.0% in controls), but Kuyumcu et al. (2012) (66) found a lower prevalence (60.6% vs 70.5% in controls).
Eleven studies reported data on the prevalence of diabetes in AD dementia and controls (Figure 2C) (37, 63–66, 69, 72, 74, 77, 78, 93). In most studies, diabetes was slightly more common in individuals with AD dementia, or prevalence was similar between the groups. The largest difference between AD dementia and controls reported was in Lyou et al. (2018) (72) (AD dementia, 44.9%; controls, 30.1%; p < 0.0001). Of the eleven studies, only Kuyumcu et al. (2012) (66) found a lower prevalence of diabetes in AD dementia than in controls (18.1% vs 23.1%).
Eight studies reported data on stroke (Figure 2D) (37, 63, 65, 71, 74, 76, 77, 93). In two studies, the prevalence of stroke was similar in people with AD dementia and in controls (37, 74), in three studies stroke was slightly more common in people with AD dementia (p < 0.001 in all three studies) (65, 77, 93), and in one study, stroke was considerably more common in people with AD dementia than in controls (17.6% vs 5.5%), but this difference was not statistically significant in a model adjusted for age and sex (63). Two studies reported hazard ratios rather than prevalence. Lee et al. (2019b) (71) found that people with AD dementia were at greater risk of stroke than controls (hazard ratio: 2.87 [95% confidence interval: 2.71–3.04]). Tolppanen et al. (2013c) found that hemorrhagic stroke was slightly more common in AD dementia than in controls, but ischaemic stroke was less common (Table S5) (76). In five studies, the prevalence of CVD or specific cardiovascular conditions other than stroke was higher in AD dementia cohorts than in controls (63, 66, 77, 78, 93); however, Grande et al. (2020) (37) found similar or slightly lower prevalence of cardiovascular conditions in AD dementia than in controls (Table S5).
Disability and mortality
Only one of the identified publications assessed the impact of AD dementia on disability. Moon et al. (2021) examined a cohort of approximately 6,500 South Korean people aged 65 years or older, and found that AD dementia accounted for the greatest proportion of total DALYs (33%) and YLDs (37%) attributed to all-cause MCI and all-cause dementia in this population (99).
Similarly, only one publication compared mortality between people with AD dementia and the general population. Xu et al. (2021) used Swedish Dementia Registry data to compare mortality in 10,129 individuals with AD dementia (mean age: 81 years) against age- and year-matched controls from the general Swedish population. Over 5 years, the death rate of people with AD dementia was 115.0 per 1,000 person-years, which was approximately double that of the controls (60.6 per 1,000 person-years) (60).
Discussion
This SLR was designed to characterize AD and to estimate the burden that it imposes on affected individuals. We found evidence to indicate that people with AD dementia are more likely to have CVD, including stroke, than people without AD; however, no clear pattern was apparent for hypertension. In addition, several studies indicated that diabetes is relatively more common in people with AD dementia. There is evidence that people with AD dementia have higher mortality than those without. We have identified some key gaps in the available evidence, which can be used to inform further research.
The causes of the observed relationships between comorbidities and the development or progression of AD dementia, such as shared risk factors or disease mechanisms, warrant further investigation. Published evidence implies multifacted associations between comorbidities, such as CVD and type 2 diabetes, and the development of cognitive impairment (105, 106), and more detailed longitudinal studies that track the timing of comorbidity development relative to the development and progression of AD are needed to elucidate this complex relationship further.
Only one study that reported evidence on disability in AD dementia was identified, and it did not compare AD dementia with the general population (Moon et al. 2021) (99). Although several large Global Burden of Disease analyses have shown that dementia is a major contributor to mortality and disability in older patient populations in the USA, Europe and worldwide (15, 107–109), the grouping of AD with other dementias means that its specific impact on these factors remains less well characterized. This is reflected in a general trend identified in our SLR: although we aimed to focus on studies reporting data for AD dementia or MCI due to AD, a number of the studies grouped AD with either all-cause MCI or non-AD types of dementia, and therefore not all could be used to characterize AD dementia specifically.
The results of our SLR highlight how variability across studies leads to difficulties in collating relevant data to characterize AD dementia in specific countries and on a global scale. Disparities across studies in terms of patient age, study setting, ascertainment method and staging of AD dementia, among other factors, contributed to wide variation in the results. Importantly there were disparities in the method of AD diagnosis across studies. Some studies identified people with AD dementia in retrospective data using diagnosis codes rather than clinical assessment, and most studies did not confirm AD dementia diagnoses via assessment of amyloid and tau. Although AD biomarkers are recommended as a diagnostic tool predominantly in research settings rather than in clinical practice (6, 110), there is a large and growing body of evidence indicating that biomarker assessments can influence AD dementia diagnoses in clinical contexts. It is estimated that 10–30% of individuals diagnosed with AD by experts do not show neuropathological changes consistent with AD at autopsy, in PET scans or CSF assessments (6). Use of information derived from amyloid PET scans has been shown to influence subsequent decision making in clinical management of individuals with dementia of uncertain etiology, suggesting that it has practical benefits in reaching a diagnosis (111). It is likely that some people considered to have AD dementia in the studies identified in this SLR were misdiagnosed or had other concomitant diagnoses, which should be taken into account when interpreting the results.
It is probable that many of the prevalence and incidence data identified in this SLR underestimate the true number of individuals affected by AD, in part because few examined MCI due to AD specifically (as opposed to all-cause MCI) or confirmed AD pathology using biomarkers. A recent SLR and modeling study combined data from meta-analyses estimating the prevalence of MCI, AD dementia, and beta-amyloid positivity to calculate global estimates for biomarker-positive AD (112). Overall, it was estimated that 22% of people worldwide aged 50 years or older have AD dementia (1.7%), MCI due to AD (termed ‘prodromal AD’; 3.7%) or preclinical AD (defined as beta-amyloid positivity in people with normal cognition or subjective cognitive impairment; 17%) (112). Although the estimate for AD dementia prevalence falls within the range identified in our SLR, the larger estimate when preclinical AD and MCI due to AD are taken into account highlights the potential burden of AD that is not captured by studies including only individuals with a formal diagnosis of AD dementia.
The previously published modeling study indicated that estimation of the worldwide impact of AD is compromised by the paucity of data in some geographical regions. This is supported by the findings of our SLR. European and Asian data were drawn only from subsets of countries, and North American data were only from the USA. Data from South America, Africa, the Middle East, and Oceania were few or absent.
A requirement for mention or specification of AD stage or severity in the publication title or abstract was included in the SLR design to maintain focus on AD, and to identify evidence on the impact of AD progression. To identify the most recent evidence, data extraction was restricted to publications from 2015 onwards. Although a wide range of outcomes were included, it is likely that some relevant evidence was not identified in searches owing to the absence of key terms in the title or abstract. Comorbidities were examined as a broad outcome, and search terms for specific comorbidities were not included. Future, focused reviews examining specific comorbidities of interest would be highly valuable, and new studies and literature reviews should also capture the impact of the COVID-19 pandemic on care and outcomes in AD. Research is needed to investigate the biological interplay between these two diseases, as well as the effect that the sociological changes imposed by the pandemic have had on diagnosis, treatment, and support for people affected by AD dementia.
In conclusion, we have found evidence that AD is associated with morbidity, including CVD, stroke, and diabetes, and mortality. Further research in older populations is needed to quantify these risks and to understand the factors underlying AD dementia development, progression, and outcomes. Particularly given the emergence of disease modifying therapies, future longitudinal studies of population aging will be vital to track the disease course and its association with other conditions. To provide maximum value to clinicians, patients, and researchers, such studies should differentiate AD dementia from other dementias by using best practice for diagnosis and staging, incorporating assessment of biomarkers along with clinical examination and cognitive testing.
References
Nichols E, Vos T. The estimation of the global prevalence of dementia from 1990–2019 and forecasted prevalence through 2050: An analysis for the Global Burden of Disease (GBD) study 2019. Alzheimers Dement. 2021;17(Suppl 10):e051496. https://doi.org/10.1002/alz.051496
Alzheimer’s Association. What is Alzheimer’s Disease? Available at: https://www.alz.org/alzheimers-dementia/what-is-alzheimers. (Accessed 2 Oct 2022).
Selkoe DJ, LansburyJr. PJ. Alzheimer’s Disease Is the Most Common Neurodegenerative Disorder. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Siegel GJ, Agranoff BW, Albers RW, et al., editors. Philadelphia: Lippincott-Raven. Basic Neurochemistry: Molecular, Cellular and Medical Aspect. 1999
Alzheimers.gov. What Is Mild Cognitive Impairment?. 2022. Available at: https://www.alzheimers.gov/alzheimers-dementias/mild-cognitive-impairment (Accessed 18 Jan 2023).
Alzheimers.gov. What Is Dementia? 2022. Available at: https://www.alzheimers.gov/alzheimers-dementias/what-is-dementia (Accessed 18 Jan 2023).
Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Davis M, O’Connell T, Johnson S, et al. Estimating Alzheimer’s disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. 2018;15:777–788. 10.2174/1567205015666180119092427
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. https://doi.org/10.1016/j.jalz.2011.03.003
National Institute on Aging. Alzheimer’s Disease Diagnostic Guidelines. 2022. Available at: https://www.nia.nih.gov/health/alzheimers-disease-diagnostic-guidelines (Accessed 18 Jan 2023).
Food and Drug Administration. Drug Approval Label: Leqembi (lecanemabirmb). 2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269Orig1s000lbl.pdf (Accessed 26 Jan 2023).
Food and Drug Administration. Drug Approval Package: Aduhelm (aducanumab-avwa). 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761178Orig1s000TOC.cfm (Accessed 31 Oct 2022).
Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406. https://doi.org/10.1002/alz.12328
Newcombe EA, Camats-Perna J, Silva ML, et al. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation. 2018;15:276. https://doi.org/10.1186/s12974-018-1313-3
Santiago JA, Potashkin JA. The impact of disease comorbidities in Alzheimer’s disease. Front Aging Neurosci. 2021;13:631770. https://doi.org/10.3389/fnagi.2021.631770
GBD Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. https://doi.org/10.1016/S1474-4422(18)30403-4
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535
Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol. 2017;81:549–559. https://doi.org/10.1002/ana.24903
Akushevich I, Kravchenko J, Yashkin AP, Yashin AI. Time trends in the prevalence of cancer and non-cancer diseases among older U.S. adults: Medicare-based analysis. Exp Gerontol. 2018;110:267–276. https://doi.org/10.1016/j.exger.2018.06.017
Alonso A, Knopman DS, Gottesman RF, et al. Correlates of dementia and mild cognitive impairment in patients with atrial fibrillation: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J Am Heart Assoc. 2017;6:24. https://doi.org/10.1161/JAHA.117.006014
Andreu-Reinon ME, Huerta JM, Gavrila D, et al. Incidence of dementia and associated factors in the EPIC-Spain dementia cohort. J Alzheimers Dis. 2020;78(2):543–555. https://doi.org/10.3233/JAD-200774
Assaf G, Rahme D, El Khoury J. Prevalence of mild cognitive impairment and associated risk factors in ambulatory senior patients. J Am Geriatr Soc. 2018;66(Supplement 2):S59. https://doi.org/10.1111/jgs.15376
Beauchet O, Sekhon H, Launay CP, et al. Motoric cognitive risk syndrome and incident dementia: results from a population-based prospective and observational cohort study. Eur J Neurol. 2020;27:468–474. https://doi.org/10.1111/ene.14093
Bouteloup V, Dufouil C, Mangin JF, et al. NIA-AA Research Framework: application to the French population by inference from the Memento cohort. Alzheimers Dement. 2019;15(7 Supplement):P1251. https://doi.org/10.1016/j.jalz.2019.06.4780
Chiari A, Vinceti G, Adani G, et al. Epidemiology of early onset dementia and its clinical presentations in the province of Modena, Italy. Alzheimers Dement. 2021;17:81–88. https://doi.org/10.1002/alz.12177
Chua XY, Ha NHL, Cheong CY, Wee SL, Yap PLK. The changing profile of patients in a geriatric medicine led memory clinic over 12 years. J Nutr Health Aging. 2019;23:310–315. https://doi.org/10.1007/s12603-019-1161-6
Chuang YF, Varma V, An Y, et al. Interaction between apolipoprotein E and butyrylcholinesterase genes on risk of Alzheimer’s disease in a prospective cohort study. J Alzheimers Dis. 2020;75:417–427. https://doi.org/10.3233/JAD-191335
Dang C, Harrington KD, Lim YY, et al. Relationship between amyloid-beta positivity and progression to mild cognitive impairment or dementia over 8 years in cognitively normal older adults. J Alzheimers Dis. 2018;65:1313–1325. https://doi.org/10.3233/JAD-180507
de Mauleon A, Soto M, Kiyasova V, et al. The ROSAS cohort: a prospective, longitudinal study of biomarkers for Alzheimer’s disease. Strategy, methods and initial results. J Prev Alzheimers Dis. 2017;4:183–193. https://doi.org/10.14283/jpad.2017.8
Degen C, Toro P, Frankenberg C, et al. The natural course of mild cognitive impairment: a 20 years perspective. Neurodegener Dis. 2017;17 (Supplement 1):1809.
Degerman Gunnarsson M, Ingelsson M, Blennow K, et al. High tau levels in cerebrospinal fluid predict nursing home placement and rapid progression in Alzheimer’s disease. Alzheimers Res Ther. 2016;8:22. https://doi.org/10.1186/s13195-016-0191-0
Dominguez JC, Palines JDV. Risk factors for progression of mild cognitive impairment to Alzheimer’s dementia in Filipino patients: a prospective study - a subgroup analysis from the Marikina Memory and Ageing cohort. Alzheimers Dement. 2018;14(7 Supplement):P1193. https://doi.org/10.1016/j.jalz.2018.06.1653
El Tallawy HNA, Farghaly WMA, El Hamed MA, et al. Prevalence of Alzheimer dementia in upper Egypt (desert areas). Egypt J Neurol Psychiatr Neurosurg. 2019;55(1):29 https://doi.org/10.1186/s41983-019-0074-y
Epelbaum S, Paquet C, Hugon J, et al. How many patients are eligible for disease-modifying treatment in Alzheimer’s disease? A French national observational study over 5 years. BMJ Open. 2019;9:e029663. https://doi.org/10.1136/bmjopen-2019-029663
Gallagher D, Kiss A, Lanctot KL, Herrmann N. Toward prevention of mild cognitive impairment in older adults with depression: an observational study of potentially modifiable risk factors. J Clin Psychiatry. 2018;80:27. https://doi.org/10.4088/JCP.18m12331
Gong J, Harris K, Peters SAE, Woodward M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med. 2021;19(1):110 https://doi.org/10.1186/s12916-021-01980-z
Gracner T, Stone PW, Agarwal M, et al. Advanced cognitive impairment among older nursing home residents. BMC Geriatr. 2021;21(1):382. https://doi.org/10.1186/s12877-021-02336-1
Grande G, Vetrano DL, Mazzoleni F, et al. Detection and prediction of incident Alzheimer dementia over a 10-year or longer medical history: a population-based study in primary care. Dement Geriatr Cogn Disord. 2020;49(4):384–389. https://doi.org/10.1159/000509379
Honda H. Trends in dementia prevalence over 31 years of the Hisayama study. Brain Pathol. 2019;29 (Supplement 1):64. https://doi.org/10.1111/bpa.12675
Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5:e661–e671. https://doi.org/10.1016/S2468-2667(20)30185-7
Kirson NY, Meadows ES, Desai U, et al. Temporal and geographic variation in the incidence of Alzheimer’s disease diagnosis in the US between 2007 and 2014. J Am Geriatr Soc. 2020;68(2):346–353. https://doi.org/10.1111/jgs.16262
Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement (Amst). 2016;2:1–11. https://doi.org/10.1016/j.dadm.2015.12.002
Lai F, Mhatre PG, Yang Y, et al. Sex differences in risk of Alzheimer’s disease in adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(1) (no pagination) https://doi.org/10.1002/dad2.12084
Lee KH, Kwon DE, Do Han K, La Y, Han SH. Association between cytomegalovirus end-organ diseases and moderate-to-severe dementia: a population-based cohort study. BMC Neurol. 2020;20(1):e12084 https://doi.org/10.1186/s12883-020-01776-3
Loi SM, Chen B, Tsoukra P, et al. Cognitive manifestations are a risk factor of mortality in younger-onset dementia. Alzheimers Dement. 2020;16:e12278. https://doi.org/10.1002/alz.12278
Miller AH, Marra DE, Wu Y, et al. Characterizing dementia prevalence in the state of Florida: an electronic health record study. Alzheimers Dement. 2021;17:e052364. https://doi.org/10.1002/alz.052364
Mouna A, Wafa A, Dhouha F, et al. Prevalence of dementia in a clinical population followed in psychiatry in the southern of Tunisia. J Neurol Sci. 2019;405(Supplement):143. https://doi.org/10.1016/j.jns.2019.10.1043
Peeters G, Katelekha K, Lawlor B, Demnitz N. Sex differences in the incidence and prevalence of young-onset Alzheimer’s disease: a meta-analysis. Int J Geriatr Psychiatry. 2021;12:12. https://doi.org/10.1002/gps.5612
Petersen MS, Restorff M, Stora T, Waldemar G, Joensen S. Trend in the incidence and prevalence of dementia in the Faroe Islands. J Alzheimers Dis. 2019;71:969–978. https://doi.org/10.3233/JAD-190341
Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement. 2019;15:1–7. https://doi.org/10.1016/j.jalz.2018.07.216
Ren D, Lopez OL, Lingler JH, Conley Y. The effect of the APOE epsilon2epsilon4 genotype on the development of Alzheimer’s disease (AD) and mild cognitive impairment (MCI) in non-Latino Whites. J Am Geriatr Soc. 2020;68:1044–1049. https://doi.org/10.1111/jgs.16337
Ren D, Lopez OL, Lingler JH, Conley Y. Association between the APOE 2/4 genotype and Alzheimer’s disease and mild cognitive impairment among African Americans. J Alzheimers Dis. 2021;81(3):943–948. https://doi.org/10.3233/JAD-201613
Ruano L, Araujo N, Branco M, et al. Prevalence and causes of cognitive impairment and dementia in a population-based cohort from northern Portugal. Am J Alzheimers Dis Other Demen. 2019;34:49–56. https://doi.org/10.1177/1533317518813550
Slot RER, Sikkes SAM, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimers Dement. 2019;15:465–476. https://doi.org/10.1016/j.jalz.2018.10.003
Souza RKM, Barboza AF, Gasperin G, et al. Prevalence of dementia in patients seen at a private hospital in the southern region of Brazil. Einstein (Sao Paulo). 2019;18:eAO4752. https://doi.org/10.31744/einstein_journal/2020AO4752
Sutovsky S, Kralova M, Siarnik P, Turcani P. Prevalence, recognition, and treatment of dementia in assisted living facilities. Dement Geriatr Cogn Disord. 2018;45:27–37. https://doi.org/10.1159/000487129
Vlachos GS, Kosmidis MH, Yannakoulia M, et al. Prevalence of mild cognitive impairment in the elderly population in Greece: results from the HELIAD study. Alzheimer Dis Assoc Disord. 2020;34:156–162. https://doi.org/10.1097/WAD.0000000000000361
Vlachos GS, Kosmidis MH, Yannakoulia M, et al. Incidence of mild cognitive impairment in the elderly population in Greece: results from the HELIAD study. Aging Clin Exp Res. 2021;33(10):2679–2688. https://doi.org/10.1007/s40520-021-01819-w
Vlachos GS, Kosmidis MH, Yannakoulia M, et al. Dementia incidence in the elderly population of Greece: results from the HELIAD study. Alzheimer Dis Assoc Disord. 2021;35(1):48–54. https://doi.org/10.1097/WAD.0000000000000407
Wan Suh S, Kim YJ, Kwak KP, et al. A 9-year comparison of dementia prevalence in Korea: results of NaSDEK 2008 and 2017. J Alzheimers Dis. 2021;03 https://doi.org/10.3233/JAD-201588
Xu H, Garcia-Ptacek S, Jonsson L, et al. Long-term effects of cholinesterase inhibitors on cognitive decline and mortality. Neurology. 2021;96(17):e2220–e2230. https://doi.org/10.1212/WNL.0000000000011832
Yuan J, Maserejian N, Liu Y, et al. Severity distribution of Alzheimer’s disease dementia and mild cognitive impairment in the Framingham Heart Study. J Alzheimers Dis. 2021;79(2):807–817. https://doi.org/10.3233/JAD-200786
Yuan J, Zhang Z, Wen H, et al. Incidence of dementia and subtypes: a cohort study in four regions in China. Alzheimers Dement. 2016;12:262–271. https://doi.org/10.1016/j.jalz.2015.02.011
Andersen F, Viitanen M, Halvorsen DS, Straume B, Engstad TA. Co-morbidity and drug treatment in Alzheimer’s disease. A cross sectional study of participants in the dementia study in northern Norway. BMC Geriatr. 2011;11:58. https://doi.org/10.1186/1471-2318-11-58
de Lima JD, Teixeira IA, Silva FO, Deslandes AC. The comorbidity conditions and polypharmacy in elderly patients with mental illness in a middle income country: a cross-sectional study. IBRO Rep. 2020;9:96–101. https://doi.org/10.1016/j.ibror.2020.07.008
Ilmaniemi S, Taipale H, Tanskanen A, et al. Incidence of head injury and traumatic brain injury among people with Alzheimer’s disease. J Epidemiol Community Health. 2019;73:451–454. https://doi.org/10.1136/jech-2018-211960
Kuyumcu ME, Yesil Y, Ozturk ZA, et al. Alzheimer’s disease is associated with a low prevalence of hypertension. Dement Geriatr Cogn Disord. 2012;33:6–10. https://doi.org/10.1159/000336053
Lanyau-Dominguez Y, Macias-Matos C, Jesus J, et al. Levels of vitamins and homocysteine in older adults with Alzheimer disease or mild cognitive impairment in Cuba. MEDICC Rev. 2020;22:40–47. https://doi.org/10.37757/MR2020.V22.N4.14
Lee H. Urge incontinence in Alzheimer’s disease: a population-based cohort study in Taiwan. Neurourol Urodyn. 2016;35:S249.
Lee HY, Li CC, Juan YS, et al. Urinary incontinence in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2017;32:51–55. https://doi.org/10.1177/1533317516680900
Lee JH. Association of Alzheimer’s disease with the risk of developing epilepsy: a 10-year nationwide cohort study. Alzheimers Dement. 2019;15:P695. https://doi.org/10.1016/j.jalz.2019.06.2691
Lee JH. Risk of stroke in patients with Alzheimer’s disease. J Prev Alzheimers Dis. 2019;6:S140–S141.
Lyou HJ, Seo KD, Lee JE, Pak HY, Lee JH. Association of Alzheimer’s disease with the risk of developing epilepsy: a 10-year nationwide cohort study. Dement Neurocogn Disord. 2018;17:156–162. https://doi.org/10.12779/dnd.2018.17.4.156
Sannemann L, Schild AK, Altenstein S, et al. Neuropsychiatric symptoms in at-risk groups for AD dementia and their association with worry and AD biomarkers—results from the DELCODE study. Alzheimers Res Ther. 2020;12:131. https://doi.org/10.1186/s13195-020-00701-7
Tolppanen AM, Kettunen R, Ahonen R, Soininen H, Hartikainen S. Incident ischaemic heart disease in persons with Alzheimer’s disease in a Finnish nationwide exposure-matched cohort. Int J Cardiol. 2013;170:195–201. https://doi.org/10.1016/j.ijcard.2013.10.065
Tolppanen AM, Lavikainen P, Soininen H, Hartikainen S. Incident hip fractures among community dwelling persons with Alzheimer’s disease in a Finnish nationwide register-based cohort. PLoS One. 2013;8:e59124. https://doi.org/10.1371/journal.pone.0059124
Tolppanen AM, Lavikainen P, Solomon A, et al. Incidence of stroke in people with Alzheimer disease: a national register-based approach. Neurology. 2013;80:353–358. https://doi.org/10.1212/WNL.0b013e31827f08c5
Tolppanen AM, Taipale H, Tanskanen A, Tiihonen J, Hartikainen S. Comparison of predictors of hip fracture and mortality after hip fracture in community-dwellers with and without Alzheimer’s disease - exposure-matched cohort study. BMC Geriatr. 2016;16:204. https://doi.org/10.1186/s12877-016-0383-2
Wang JH, Wu YJ, Tee BL, Lo RY. Medical comorbidity in Alzheimer’s disease: a nested case-control study. J Alzheimers Dis. 2018;63:773–781. https://doi.org/10.3233/JAD-170786
Yazar T, Olgun Yazar H. The prevalence of sarcopenia and dynapenia according to stage among Alzheimer-type dementia patients. Ideggyogy Sz. 2019;72:171–179. https://doi.org/10.18071/isz.72.0171
Chen KH, Chen HH, Li L, et al. The impact of exercise on patients with dementia: a 2-year follow-up. Medicine (Baltimore). 2020;99:e20597. https://doi.org/10.1097/MD.0000000000020597
Chen TB, Weng SC, Chou YY, et al. Predictors of mortality in the oldest old patients with newly diagnosed Alzheimer disease in a residential aged care facility. Dement Geriatr Cogn Disord. 2019;48:93–104. https://doi.org/10.1159/000503059
Dutkiewicz R, Zetterberg H, Andreasson U, Blennow K, Nellgard B. Dementia and CSF-biomarkers for Alzheimer’s disease predict mortality after acute hip fracture. Acta Anaesthesiol Scand. 2020;64:93–103. https://doi.org/10.1111/aas.13472
Hirose D, Hanyu H, Fukasawa R, et al. Frailty and sarcopenia in subjects with Alzheimer’s disease with or without cerebrovascular disease. Geriatr Gerontol Int. 2016;16:1235–1236. https://doi.org/10.1111/ggi.12709
Kelaiditi E, Andrieu S, Cantet C, et al. Frailty index and incident mortality, hospitalization, and institutionalization in Alzheimer’s disease: data from the ICTUS study. J Gerontol A Biol Sci Med Sci. 2016;71:543–548. https://doi.org/10.1093/gerona/glv137
Ku LJE, Li CY, Sun Y. Can persistence with cholinesterase inhibitor treatment lower mortality and health-care costs among patients with Alzheimer’s disease? A population-based study in Taiwan. Am J Alzheimers Dis Other Demen. 2018;33:86–92. https://doi.org/10.1177/1533317517734639
Manso-Calderon R, Cacabelos-Perez P, Sevillano-Garcia MD, Herrero-Prieto ME, Gonzalez-Sarmiento R. The impact of vascular burden on behavioural and psychological symptoms in older adults with dementia: the BEVASDE study. Neurol Sci. 2020;41(1):165–174. https://doi.org/10.1007/s10072-019-04071-3
Mueller C, Huntley J, Stubbs B, et al. Associations of neuropsychiatric symptoms and antidepressant prescription with survival in Alzheimer’s disease. J Am Med Dir Assoc. 2017;18:1076–1081. https://doi.org/10.1016/j.jamda.2017.07.001
Mueller C, Perera G, Hayes RD, Shetty H, Stewart R. Associations of acetylcholinesterase inhibitor treatment with reduced mortality in Alzheimer’s disease: a retrospective survival analysis. Age Ageing. 2018;47:88–94. https://doi.org/10.1093/ageing/afx098
Pan CC, Chu CS, Chen CL, Chuang YC, Chen NC. Factors affecting rapid cognitive decline in patients with Alzheimer’s disease: a longitudinal follow-up study. Int J Environ Res Public Health. 2021;18(16):8576 https://doi.org/10.3390/ijerph18168576
Rhodius-Meester HFM, Liedes H, Koene T, et al. Disease-related determinants are associated with mortality in dementia due to Alzheimer’s disease. Alzheimers Res Ther. 2018;10:23. https://doi.org/10.1186/s13195-018-0348-0
Secnik J, Schwertner E, Alvarsson M, et al. Cholinesterase inhibitors in patients with diabetes mellitus and dementia: an open-cohort study of ∼23 000 patients from the Swedish Dementia Registry. BMJ Open Diabetes Res. Care. 2020;8:e000833. https://doi.org/10.1136/bmjdrc-2019-000833
Staekenborg SS, Pijnenburg YAL, Lemstra AW, Scheltens P, Vd Flier WM. Dementia and rapid mortality: who is at risk? J Alzheimers Dis. 2016;53:135–142. https://doi.org/10.3233/JAD-151063
Vu M, Koponen M, Taipale H, et al. Prevalence of cardiovascular drug use before and after diagnosis of Alzheimer’s disease. Int J Cardiol. 2020;300:221–225. https://doi.org/10.1016/j.ijcard.2019.09.036
Yeh TS, Wang JD, Ku LE. Estimating life expectancy and lifetime healthcare costs for Alzheimer’s disease in Taiwan: does the age of disease onset matter? J Alzheimers Dis. 2020;73:307–315. https://doi.org/10.3233/JAD-181060
Garre-Olmo J, Ponjoan A, Inoriza JM, et al. Survival, effect measures, and impact numbers after dementia diagnosis: a matched cohort study. Clin Epidemiol. 2019;11:525–542. https://doi.org/10.2147/CLEP.S213228
Haaksma ML, Eriksdotter M, Rizzuto D, et al. Survival time tool to guide care planning in people with dementia. Neurology. 2020;94:e538–e548. https://doi.org/10.1212/WNL.0000000000008745
Hager K, Baseman AS, Nye JS, et al. Effect of concomitant use of memantine on mortality and efficacy outcomes of galantamine-treated patients with Alzheimer’s disease: post-hoc analysis of a randomized placebo-controlled study. Alzheimers Res Ther. 2016;8:1–10. https://doi.org/10.1186/s13195-016-0214-x
Mank A, Van Maurik IS, Bakker ED, et al. Predicting institutionalization and mortality across the spectrum of Alzheimer’s disease. Alzheimers Dement. 2021;17:e051407. https://doi.org/10.1002/alz.051407
Moon W, Han JW, Bae JB, et al. Disease burdens of Alzheimer’s disease, vascular dementia, and mild cognitive impairment. J Am Med Dir Assoc. 2021;22(10):2093–2099.e3. https://doi.org/10.1016/j.jamda.2021.05.040
Patel H, Kielhorn A, Yurgin N, Hernandez A. Years of life lost due to heart failure in the United States (US). Circulation. 2015;132:A17368. https://doi.org/10.1161/circ.132.suppl_3.17368
Rhodius-Meester HF, Tijms BM, Lemstra E, et al. Median survival in memory clinic cohort is short, even in young-onset dementia. Alzheimers Dement. 2018;14(7 Supplement):P431. https://doi.org/10.1016/j.jalz.2018.06.365
Saad AMM, Alfaar AS. 434P - Is radiation therapy influencing Alzheimer’s in brain and head and neck cancers? Ann Oncol. 2019;30:v157. https://doi.org/10.1093/annonc/mdz243.044
Schmutte T, Olfson M, Maust DT, Xie M, Marcus SC. Suicide risk in first year after dementia diagnosis in older adults. Alzheimers Dement. 2022;18(2):262–271.; https://doi.org/10.1002/alz.12390
Vazquez Justes D, Dakterzada F, Romero L, et al. Microbleeds and the risk of stroke and mortality in patients with alzheimer disease after 3 years of follow-up. Eur Stroke J. 2021;6(1 Supplement):443. https://doi.org/10.1177/23969873211034932
Zheng B, Su B, Udeh-Momoh C, et al. Associations of cardiovascular and non-cardiovascular comorbidities with dementia risk in patients with diabetes: results from a large UK cohort study. J Prev Alzheimers Dis. 2022;9:86–91. https://doi.org/10.14283/jpad.2022.8
Zuo W, Wu J. The interaction and pathogenesis between cognitive impairment and common cardiovascular diseases in the elderly. Ther Adv Chronic Dis. 2022;13:20406223211063020. https://doi.org/10.1177/20406223211063020
GBD US Neurological Disorders Collaborators, Feigin VL, Vos T, et al. Burden of neurological disorders across the US from 1990–2017: A Global Burden of Disease study. JAMA Neurol. 2021;78:165–176. https://doi.org/10.1001/jamaneurol.2020.4152
Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5:e551–e567. https://doi.org/10.1016/S2468-2667(20)30190-0
GBD Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. https://doi.org/10.1016/S1474-4422(17)30299-5
Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. 10.1016/S1474-4422(14)70090-0
Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286–1294. https://doi.org/10.1001/jama.2019.2000
Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 2022; https://doi.org/10.1002/alz.12694
Acknowledgments
Krista L. Lanctôt is supported by the Bernick Chair in Geriatric Psychopharmacology, NIA grant 1R01AG068324 and the Alzheimer’s Drug Discovery Foundation. Jeffrey Cummings is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; NIA grant P20AG068053; NIA grant P30AG072959; NIA grant R35AG71476; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; and the Joy Chambers-Grundy Endowment.
Funding
Funding: This SLR was funded by Novo Nordisk A/S, who contributed to the design of the SLR, data interpretation and manuscript review and approval. The SLR and data analysis, and manuscript preparation, were conducted by Oxford PharmaGenesis, Oxford, UK with funding from Novo Nordisk A/S.
Author information
Authors and Affiliations
Contributions
Author contributions: Julie Hviid Hahn-Pedersen, Christian Eichinger, and Caroline Freeman designed the original SLR. Christian Eichinger and Caroline Freeman conducted the SLR. All authors contributed to subsequent data synthesis and interpretation, and critical review and revision of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest: Krista L. Lanctôt has acted as an adviser/consultant for BioXcel Therapeutics, Bright Minds Biosciences, Cerevel Therapeutics, Eisai, Exciva,, ICG Pharma, Jazz Pharmaceuticals Kondor Pharma, Lundbeck, Merck, Novo Nordisk A/S, Praxis Precision Medicines, and Sumitomo Pharma. Julie Hviid Hahn-Pedersen, Alice Clark, and Luis Rafael Solís Tarazona are employees of Novo Nordisk A/S. Christian Stefan Eichinger and Caroline Freeman are employees of Oxford PharmaGenesis, which received funding for conducting the SLR. Jeffrey Cummings has provided consultation to Acadia, Alkahest, AlphaCognition, AriBio, Biogen, Cassava Sciences, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna Therapeutics, Lilly, LSP, Merck, NervGen Pharma, Novo Nordisk A/S, Oligomerix, Ono, Otsuka Pharmaceutical, PRODEO, Prothena, ReMYND, Resverlogix, Roche, Signant Health, Suven Pharmaceuticals, and United Neuroscience pharmaceutical, assessment, and investment companies.
Electronic supplementary material
42414_2023_61_MOESM1_ESM.docx
Burden of Illness in People with Alzheimer’s Disease: A Systematic Review of Epidemiology, Comorbidities and Mortality, approximately 488 KB.
Rights and permissions
Open Access : This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Lanctôt, K.L., Hahn-Pedersen, J.H., Eichinger, C.S. et al. Burden of Illness in People with Alzheimer’s Disease: A Systematic Review of Epidemiology, Comorbidities and Mortality. J Prev Alzheimers Dis 11, 97–107 (2024). https://doi.org/10.14283/jpad.2023.61
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.61