Abstract
Background
Increasing evidence indicates that β-secretase 1 (BACE1) activity and concentration in blood are candidate biomarkers for Alzheimer’s disease (AD). Investigating potential demographic, biological, and clinical determinants of BACE1 in the blood matrix is the critical step to validate and qualify BACE1 bio-indicators for different contexts-of-use (CoU), such as risk assessment, early detection, diagnosis, prognosis, management of AD, and outcome of amyloid pathway targeted drugs.
Objectives
To evaluate the influence of age, sex, HDL-cholesterol and comorbidities (cardiovascular diseases, hypertension, diabetes) on circulating BACE-1 activity.
Design
prospective analysis of serum samples, clinical, biological, and demographic variables.
Setting
Three cohorts: 1) Memory Clinic of the Department of Internal Medicine, S. Anna University Hospital, Ferrara (Italy); 2) outpatients attending the Menopause and Osteoporosis Centre (MOC) of the University of Ferrara (Ferrara, Italy); 3) Prevention Center of the University of Ferrara. Participants: 504 cognitively healthy individuals (median age: 62 years, interquartile range: 51–73) and 175 patients with AD (78 years, 74–82).
Measurements
serum BACE1 (sBACE1), age, sex, HDL-cholesterol, major comorbidities.
Results
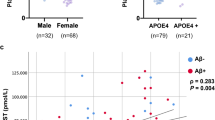
Age was the strongest independent predictor of sBACE1 variance (β=0.425, p<0.0001), followed by sex (β=0.180, p<0.0001), high density lipoprotein-cholesterol (HDL-C) (β=−0.168, p<0.0001) and hypertension (β=0.111, p<0.05) (overall model, R2: 0.232). The probability of having elevated sBACE1 activity increased after 70 years of age, with women being more susceptible to higher sBACE1 activity than men.
Conclusions
We provide evidence about potential clinical and biological determinants of sBACE1 activity with a strong association among biomarker, female sex, and older age.



Similar content being viewed by others
References
Hampel H, Cummings J, Blennow K, et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol 2021;17:580–589. https://doi.org/10.1038/s41582-021-00520-w
Zetterberg H. Blood-based biomarkers for Alzheimer’s disease-An update. J Neurosci Methods 2019;319:2–6. https://doi.org/10.1016/j.jneumeth.2018.10.025
Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Cervellati C, Trentini A, Rosta V, et al. Serum beta-secretase 1 (BACE1) activity as candidate biomarker for late-onset Alzheimer’s disease. Geroscience 2020;42:159–167. https://doi.org/10.1007/s11357-019-00127-6
Zuliani G, Trentini A, Rosta V, et al. Increased blood BACE1 activity as a potential common pathogenic factor of vascular dementia and late onset Alzheimer’s disease. Sci Rep 2020;10:14980. https://doi.org/10.1038/s41598-020-72168-3
Zuliani G, Trentini A, Brombo G, et al. Serum beta-secretase 1 (BACE1) activity increases in patients with mild cognitive impairment. J Neurochem 2021;159:629–637. https://doi.org/10.1111/jnc.15513
Shen Y, Wang H, Sun Q, et al. Increased Plasma Beta-Secretase 1 May Predict Conversion to Alzheimer’s Disease Dementia in Individuals With Mild Cognitive Impairment. Biol Psychiat 2018;83:447–455. https://doi.org/10.1016/j.biopsych.2017.02.007
Mulder SD, Van Der Flier WM, Verheijen JH, et al. BACE1 activity in cerebrospinal fluid and its relation to markers of AD pathology. J Alzheimers Dis 2010;20(1):253–60. doi: https://doi.org/10.3233/JAD-2010-1367
Ewers M, Cheng X, Zhong Z, et al. Increased CSF-BACE1 Activity Associated with Decreased Hippocampus Volume in Alzheimer’s Disease. J Alzheimers Dis 2011;25:373–381. https://doi.org/10.3233/JAD-2011-091153
Wu G, Sankaranarayanan S, Wong J, et al. Characterization of plasma β-secretase (BACE1) activity and soluble amyloid precursor proteins as potential biomarkers for Alzheimer’s disease. J Neurosci Res 2012;90:2247–58. https://doi.org/10.1002/jnr.23122
Fukumoto H. β-Secretase Protein and Activity Are Increased in the Neocortex in Alzheimer Disease. Arch Neurol 2002;59:1381. https://doi.org/10.1001/archneur.59.9.1381
Stockley JH, O’Neill C. Understanding BACE1: essential protease for amyloid-β production in Alzheimer’s disease. Cell Mol Life Sci 2008;65:3265–3289. https://doi.org/10.1007/s00018-008-8271-3
Hampel H, Vassar R, De Strooper B, et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol Psychiat 2020;S0006–3223:. https://doi.org/10.1016/j.biopsych.2020.02.001
Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem 2009;110:1129–34. https://doi.org/10.1111/j.1471-4159.2009.06181.x
Hampel H, Hardy J, Blennow K, et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatr 2021;26:5481–5503. https://doi.org/10.1038/s41380-021-01249-0
Vergallo A, Houot M, Cavedo E, et al. Brain Aβ load association and sexual dimorphism of plasma BACE1 concentrations in cognitively normal individuals at risk for AD. Alzheimers Dement 2019;15:1274–1285. https://doi.org/10.1016/j.jalz.2019.07.001
Aisen PS, Cummings J, Jack CR, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther 2017;9:60. https://doi.org/10.1186/s13195-017-0283-5
Zetterberg H, Andreasson U, Hansson O, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 2008;65:1102–7. https://doi.org/10.1001/archneur.65.8.1102
Vergallo A, Lemercier P, Cavedo E, et al. Plasma β-secretase1 concentrations correlate with basal forebrain atrophy and neurodegeneration in cognitively healthy individuals at risk for AD. Alzheimers Dement 2021;17:629–640. https://doi.org/10.1002/alz.12228
Vergallo A, Houot M, Cavedo E, et al. Brain Aβ load association and sexual dimorphism of plasma BACE1 concentrations in cognitively normal individuals at risk for AD. Alzheimers Dement 2019;15:1274–1285. https://doi.org/10.1016/j.jalz.2019.07.001
Chan AS, Choi M-K, Salmon DP. The Effects of Age, Education, and Gender on the Mattis Dementia Rating Scale Performance of Elderly Chinese and American Individuals. J Gerontol B Psychol Sci Soc Sci 2001;56:P356–P363. https://doi.org/10.1093/geronb/56.6.P356
Riedel BC, Thompson PM, Brinton RD; Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol 2016;160:134–147. https://doi.org/10.1016/j.jsbmb.2016.03.012
Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality: The Women’s Health Initiative Randomized Trials. JAMA 2017;318:927–938. https://doi.org/10.1001/jama.2017.11217
Ferretti MT, Iulita MF, Cavedo E, et al. Sex differences in Alzheimer disease — the gateway to precision medicine. Nat Rev Neurol 2018;14:457–469. https://doi.org/10.1038/s41582-018-0032-9
Ewers M, Zhong Z, Bürger K, et al. Increased CSF-BACE 1 activity is associated with ApoE-ε4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain 2008;131:1252–1258. https://doi.org/10.1093/brain/awn034
Nyarko JNK, Quartey MO, Pennington PR, et al. Profiles of β-Amyloid Peptides and Key Secretases in Brain Autopsy Samples Differ with Sex and APOE ε4 Status: Impact for Risk and Progression of Alzheimer Disease. Neuroscience 2018;373:20–36. https://doi.org/10.1016/j.neuroscience.2018.01.005
Devi L, Alldred MJ, Ginsberg SD, Ohno M. Sex- and brain region-specific acceleration of β-amyloidogenesis following behavioral stress in a mouse model of Alzheimer’s disease. Mol Brain 2010;3:34. https://doi.org/10.1186/1756-6606-3-34
Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ 1994;308:1604–8. https://doi.org/10.1136/bmj.308.6944.1604
de la Torre JC. Alzheimer Disease as a Vascular Disorder. Stroke 2002;33:1152–1162. https://doi.org/10.1161/01.STR.0000014421.15948.67
Tini G, Scagliola R, Monacelli F, et al. Alzheimer’s Disease and Cardiovascular Disease: A Particular Association. Cardiol Res Pract 2020:2617970. https://doi.org/10.1155/2020/2617970
Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018;134:226–239. https://doi.org/10.1016/j.neuropharm.2017.12.030
Tamagno E, Bardini P, Obbili A, et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 2002;10:279–88. https://doi.org/10.1006/nbdi.2002.0515
Hampel H, Lista S, Neri C, Vergallo A. Time for the systems-level integration of aging: Resilience enhancing strategies to prevent Alzheimer’s disease. Prog Neurobiol. 2019;181:101662. doi: https://doi.org/10.1016/j.pneurobio.2019.101662
Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2019;92(2):84–93. doi: https://doi.org/10.1212/WNL.0000000000006745
Hampel H, Lista S, Vanmechelen E, et al. β-Secretase1 biological markers for Alzheimer’s disease: state-of-art of validation and qualification. Alzheimers Res Ther 2020;12:130. https://doi.org/10.1186/s13195-020-00686-3
Funding
Funding: No specific funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest Disclosure: All the authors declare no competing financial interests related to the present article.
Ethical standards: All studies were conducted in accordance with the Declaration of Helsinki, and local regulatory requirements. The study protocols were approved by an independent ethics committee. All patients, or next of keen, provided written consent before the start of the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cervellati, C., Vergallo, A., Trentini, A. et al. Age, Sex, Hypertension and HDL-C Alter Serum BACE1 Activity in Cognitively Normal Subjects: Implications for Alzheimer’s Disease. J Prev Alzheimers Dis 9, 708–714 (2022). https://doi.org/10.14283/jpad.2022.78
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2022.78