Abstract
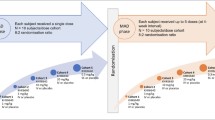
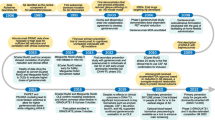
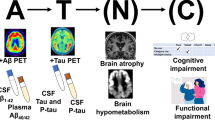
The engineered fusion protein NPT088 targets amyloid in vitro and in animal models of Alzheimer’s disease. Previous studies showed that NPT088 treatment reduced β-amyloid plaque and tau aggregate loads in mouse disease models. Here, we present the results from an initial clinical study of NPT088 in patients with mild to moderate Alzheimer’s disease. Patients were treated with 4 dose levels of NPT088 for 6 months to evaluate its safety and tolerability. Exploratory measurements included measurement of change in β-amyloid plaque and tau burden utilizing Positron Emission Tomography imaging as well as measures of Alzheimer’s disease symptoms. At endpoint NPT088 was generally safe and well-tolerated with the most prominent finding being infusion reactions in a minority of patients. No effect of NPT088 on brain plaques, tau aggregates or Alzheimer’s disease symptoms was observed.

Similar content being viewed by others
References
Toyama BH, Weissman JS. Amyloid structure: conformational diversity and consequences. Ann Rev Biochem 2011; 80: 557–85.
Krishnan R, Tsubery H, Proschitsky MY, Asp E, Lulu M, Gilead S, Gartner M, Waltho JP, Davis PJ, Hounslow AM, Kirschner DA, Inouye H, Myszka DG, Wright J, Solomon B, Fisher RA. A bacteriophage capsid protein provides a general amyloid interaction motif (GAIM) that binds and remodels misfolded protein assemblies. J Mol Biol 2014; 426:2500–19
Asp E, Proschitsky M, Lulu M, Rockwell-Postel C, Tsubery H, Krishnan R. Stability and inter-domain interactions modulate amyloid binding activity of a General Amyloid Interaction Motif. J Mol Biol 2019; 431:1920–39.
Levenson JM, Schroeter S, Carroll JC, Cullen V, Asp E, Proschitsky M, Chung CH, Gilead S, Nadeem M, Dodiya HB, Shoaga S, Mufson EJ, Tsubery H, Krishnan R, Wright J, Solomon B, Fisher R, Gannon KS. NPT088 reduces both amyloid-β and tau pathologies in transgenic mice. Alzheimers Dement 2016; 2:141–155.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7:263–9.
Doraiswamy PM, Sperling RA, Johnson KA, Reiman EM, Davis MD, Grundman M, Sabbagh MN, Sadowsky CH, Fleisher AS, Carpenter A, Clark CM, Joshi AD, Mintun MA, Skovronsky DM, Pontecorvo MJ. Amyloid b assessed by florbetapir F18 PET and 18 month cognitive decline. Neurology 2012; 79:1636–44.
Kroth H, Oden F, Molette J, Schieferstein H, Capotosti F, Mueller A, Berndt M, Schmitt-Willich H, Darmency V, Gabellieri E, Boudou C, Juergens T, Varisco Y, Vokali E, Hickman DT, Tamagnan G, Pfeifer A, Dinkelborg L, Muhs A, Stephens A. Discovery and preclinical characterization of [18F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur J Nucl Med Mol Imaging 2019; 46:2178–89.
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016; 537(7618):50–6.
Acknowledgments
The authors wish to thank the patients, families and investigative site staff who participated in this study and without whose generous contribution of time and effort the study would not have been possible.
Funding
Funding: This study was funded by Proclara Biosciences and by Part the Cloud grant PTC-17-442898 from the Alzheimer’s Association
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosures: David Michelson, Richard Fisher, Franz Hefti, Michelle Gray, and Jonathan Levenson are all current or former employees of Proclara Biosciences. Michael Grundman and Paul Aisen were paid consultants to Proclara Biosciences. Kenneth Marek is a former employee of Invicro and has served as a paid consultant to Proclara Biosciences.
Rights and permissions
About this article
Cite this article
Michelson, D., Grundman, M., Magnuson, K. et al. Randomized, Placebo Controlled Trial of NPT088, A Phage-Derived, Amyloid-Targeted Treatment for Alzheimer’s Disease. J Prev Alzheimers Dis 6, 228–231 (2019). https://doi.org/10.14283/jpad.2019.37
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2019.37