Abstract
Background
Tesamorelin, a growth hormone-releasing hormone analogue, decreases visceral adipose tissue in people living with HIV, however, the effects on skeletal muscle fat and area are unknown.
Objectives
The goals of this exploratory secondary analysis were to determine the effects of tesamorelin on muscle quality (density) and quantity (area).
Design
Secondary, exploratory analysis of two previously completed randomized (2:1), clinical trials.
Setting
U.S. and Canadian sites.
Participants
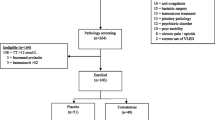
People living with HIV and with abdominal obesity. Tesamorelin participants were restricted to responders (visceral adipose tissue decrease ≥8%).
Intervention
Tesamorelin or placebo.
Measurements
Computed tomography scans (at L4-L5) were used to quantify total and lean density (Hounsfield Units, HU) and area (centimeters2) of four trunk muscle groups using a semi-automatic segmentation image analysis program. Differences between muscle area and density before and after 26 weeks of tesamorelin or placebo treatment were compared and linear regression models were adjusted for baseline and treatment arm.
Results
Tesamorelin responders (n=193) and placebo (n=148) participants with available images were similar at baseline; most were Caucasian (83%) and male (87%). In models adjusted for baseline differences and treatment arm, tesamorelin was associated with significantly greater increases in density of four truncal muscle groups (coefficient 1.56-4.86 Hounsfield units; all p<0.005), and the lean anterolateral/abdominal and rectus muscles (1.39 and 1.78 Hounsfield units; both p<0.005) compared to placebo. Significant increases were also seen in total area of the rectus and psoas muscles (0.44 and 0.46 centimeters2; p<0.005), and in the lean muscle area of all four truncal muscle groups (0.64-1.08 centimeters2; p<0.005).
Conclusions
Among those with clinically significant decrease in visceral adipose tissue on treatment, tesamorelin was effective in increasing skeletal muscle area and density. Long term effectiveness of tesamorelin among people with and without HIV, and the impact of these changes in daily life should be further studied.
Similar content being viewed by others
References
Smit M, Brinkman K, Geerlings S et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015, 15:810–818.
Gebo KA. Epidemiology of HIV and response to antiretroviral therapy in the middle aged and elderly. Aging health 2008, 4:615–627.
Schouten J, Wit FW, Stolte IG et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014, 59:1787–1797.
Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005, 352:48–62.
Robertson KR, Smurzynski M, Parsons TD et al. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids 2007, 21:1915–1921.
Lake JE. The Fat of the Matter: Obesity and Visceral Adiposity in Treated HIV Infection. Curr HIV/AIDS Rep 2017.
Natsag J, Erlandson KM, Sellmeyer DE et al. HIV Infection Is Associated with Increased Fatty Infiltration of the Thigh Muscle with Aging Independent of Fat Distribution. PLoS One 2017, 12:e0169184.
Althoff KN, Jacobson LP, Cranston RD et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014, 69:189–198.
Erlandson KM, Allshouse AA, Jankowski CM et al. Risk factors for falls in HIVinfected persons. J Acquir Immune Defic Syndr 2012, 61:484–489.
Grinspoon S. Epicardial adipose tissue and atherogenesis: EAT your heart out. AIDS 2014, 28:1679–1681.
Lake JE, Popov M, Post WS et al. Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. J Neurovirol 2017, 23:385–393.
Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis 2012, 205 Suppl 3:S383–390.
Shah K, Hilton TN, Myers L et al. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc 2012, 60:545–549.
Hunter GR, Gower BA, Kane BL. Age Related Shift in Visceral Fat. Int J Body Compos Res 2010, 8:103–108.
Nomura K, Eto M, Kojima T et al. Visceral fat accumulation and metabolic risk factor clustering in older adults. J Am Geriatr Soc 2010, 58:1658–1663.
Kwak JH, Jun DW, Lee SM et al. Lifestyle predictors of obese and non-obese patients with nonalcoholic fatty liver disease: A cross-sectional study. Clin Nutr 2017.
Nicklas BJ, Penninx BW, Cesari M et al. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol 2004, 160:741–749.
Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014, 2014:309570.
Lake JE, Stanley TL, Apovian CM et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis 2017, 64:1422–1429.
Drugs@FDA: FDA Approved Drug Products; Available at https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022505; Accessed 7/1/2018.
Spooner LM, Olin JL. Tesamorelin: a growth hormone-releasing factor analogue for HIV-associated lipodystrophy. Ann Pharmacother 2012, 46:240–247.
Falutz J, Allas S, Blot K et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med 2007, 357:2359–2370.
Falutz J, Allas S, Mamputu JC et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. Aids 2008, 22:1719–1728.
Falutz J, Potvin D, Mamputu JC et al. Effects of tesamorelin, a growth hormonereleasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr 2010, 53:311–322.
Stanley TL, Feldpausch MN, Oh J et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA 2014, 312:380–389.
Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000, 89:104–110.
Falutz J, Mamputu JC, Potvin D et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebocontrolled phase 3 trials with safety extension data. J Clin Endocrinol Metab 2010, 95:4291–4304.
Goodpaster BH, Carlson CL, Visser M et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001, 90:2157–2165.
Schafer AL, Vittinghoff E, Lang TF et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J Clin Endocrinol Metab 2010, 95:E368–372.
Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Annals of the New York Academy of Sciences 2000, 904:18–24.
Aubrey J, Esfandiari N, Baracos VE et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014, 210:489–497.
Boettcher M, Machann J, Stefan N et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging 2009, 29:1340–1345.
Visser M, Goodpaster BH, Kritchevsky SB et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005, 60:324–333.
Lang T, Cauley JA, Tylavsky F et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 2010, 25:513–519.
Anderson DE, Bean JF, Holt NE, Keel JC, Bouxsein ML. Computed tomography-based muscle attenuation and electrical impedance myography as indicators of trunk muscle strength independent of muscle size in older adults. American journal of physical medicine & rehabilitation /Association of Academic Physiatrists 2014, 93:553–561.
Hicks GE, Simonsick EM, Harris TB et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci 2005, 60:1420–1424.
Anderson DE, Quinn E, Parker E et al. Associations of Computed Tomography-Based Trunk Muscle Size and Density With Balance and Falls in Older Adults. J Gerontol A Biol Sci Med Sci 2016, 71:811–816.
Taaffe DR, Sipila S, Cheng S et al. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging 2005, 25:297–304.
Torriani M, Hadigan C, Jensen ME, Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol (1985) 2003, 95:1005–1010.
Erlandson KM, Fiorillo S, Masawi F et al. Antiretroviral initiation is associated with increased skeletal muscle area and fat content. Aids 2017, 31:1831–1838.
Dolan SE, Frontera W, Librizzi J et al. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med 2006, 166:1225–1231.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Adrian, S., Scherzinger, A., Sanyal, A. et al. The Growth Hormone Releasing Hormone Analogue, Tesamorelin, Decreases Muscle Fat and Increases Muscle Area in Adults with HIV. J Frailty Aging 8, 154–159 (2019). https://doi.org/10.14283/jfa.2018.45
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2018.45