Abstract
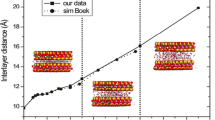
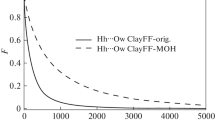
In order to understand the microscopic properties of alkylammonium-intercalated vermiculites, molecular dynamics simulations employing the clayff-CVFF force field were performed to obtain the interlayer structures and dynamics. The layering behavior of alkyl chains was uncovered. With the model used in the present study (1.2 e per unit cell), the alkyl chains formed monolayers with carbon-chain lengths of C6, bilayers from C7 to C10, and pseudo-trimolecular layers from C15 to C18. Intermediate states also existed between bilayer and pseudo-trimolecular layer states from C11 to C14. The ammonium groups had two locations: most ammonium groups were located over the six-member rings (~90%), and the rest above the substitution sites (~10%). The ammonium groups interacted with the vermiculite surface through H bonds between ammonium H atoms and surface O atoms. The ammonium groups were fixed firmly on surfaces and, therefore, had very low mobility. The alkyl chains were slightly more mobile. The similarities and differences between alkylammonium-intercalated vermiculites and smectites were revealed. The layering behaviors of alkyl chains were similar in alkylammonium-intercalated vermiculites and smectites: the alkyl chain behavior was controlled by both the amount of layer charge and the carbon chain length. The distributions of ammonium groups, however, were different, caused by the layer-charge distribution in vermiculites being different from that in smectites. The atomic-level results derived in the present study will be useful for future research into and the applications of organo-vermiculites.
Similar content being viewed by others
References
Allen, M.P. and Tildesley, D.J. (1987) Computer Simulation of Liquids. Clarendon Press, Oxford, UK.
Al-Samhan, M., Samuel, J., Al-Attar, F., and Abraham, G. (2017) Comparative effects of MMT clay modified with two different cationic surfactants on the thermal and rheological properties of polypropylene nanocomposites. International Journal of Polymer Science, 2, 1–8.
Bardziński, P.J. (2014) On the impact of intermolecular interactions between the quaternary ammonium ions on interlayer spacing of quat-intercalated montmorillonite: A molecular mechanics and ab-initio study. Applied Clay Science, 95, 323–339.
Brigatti, M.F., Galán, E., and Theng, B.K.G. (2013) Structure and mineralogy of clay minerals. Pp. 21–81 in: Handbook of Clay Science, 2nd Edition (F. Bergaya and G. Lagaly, editors). Elsevier, Amsterdam.
Carrizosa, M.J., Rice, P.J., Koskinen, W.C., Carrizosa, I., and Hermosín, M.D. (2004) Sorption of isoxaflutole and DKN on organoclays. Clays and Clay Minerals, 52, 341–349.
Chang, F.-R.C., Skipper, N.T., and Sposito, G. (1997) Monte Carlo and molecular dynamics simulations of interfacial structure in lithium-montmorillonite hydrates. Langmuir, 13, 2074–2082.
Cygan, R.T. (2001) Molecular modeling in mineralogy and geochemistry. Pp. 1–35 in: Molecular Modeling Theory: Applications in the Geosciences (R.T. Cygan and J.D. Kubicki, editors. Reviews in Mineralogy and Geochemistry, 42, Mineralogical Society of America. Washington, D.C.
Cygan, R.T., Liang, J.-J., and Kalinichev, A.G. (2004) Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. Journal of Physical Chemistry B, 108, 1255–1266.
Dauber-Osguthorpe, P., Roberts, V.A., Osguthorpe, D.J., Wolff, J., Genest, M., and Hagler, A.T. (1988) Structure and energetics of ligand binding to proteins: E. coli dihydrofolate reductasetrimethoprim, a drug-receptor system. Proteins: Structure, Function and Genetics, 4, 31–47.
Escamilla-Roa, E., Nieto, F., and Sainz-Díaz, C.I. (2016) Stability of hydronium cation in the structure of illite. Clays and Clay Minerals, 64, 413–424.
Ferrage, E. (2016) Investigation of the interlayer organization of water and ions in smectite from the combined use of diffraction experiments and molecular simulations. A review of methodology, applications, and perspectives. Clays and Clay Minerals, 64, 348–373.
Greenwell, H.C., Harvey, M.J., Boulet, P., Bowden, A.A., Coveney, P.V., and Whiting, A. (2005) Interlayer structure and bonding in nonswelling primary amine intercalated clays. Macromolecules, 38, 6189–6200.
Gruner, J.W. (1934) The structures of vermiculites and their collapse by dehydration. American Mineralogist, 19, 557–575.
Harvey, C.C. and Lagaly, G. (2013) Industrial applications. Pp. 451–490 in: Handbook of Clay Science, 2nd Edition (F. Bergaya and G. Lagaly, editors). Elsevier, Amsterdam.
He, H.P., Galy, J., and Gerard, J.F. (2005) Molecular simulation of the interlayer structure and the mobility of alkyl chains in HDTMA(+)/montmorillonite hybrids. Journal of Physical Chemistry B, 109, 13301–13306.
Heinz, H., Koerner, H., Anderson, K.L., Vaia, R.A., and Farmer, B.L. (2005) Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chemistry of Materials, 17, 5658–5669.
Janek, M. and Smrcok, L. (1999) Application of an internal standard technique by transmission X-ray diffraction to assess layer charge of a montmorillonite by using the alkylammonium method. Clays and Clay Minerals, 47, 113–118.
Jankovič, L’., Kronek, J., Madejová, J., and Hronský, V. (2015) (9, 10-Dihydroxyoctadecyl)ammonium: A structurally unique class of clay intercalable surfactants. European Journal of Inorganic Chemistry, 17, 2841–2850.
Kalinichev, A.G., Liu, X.D., and Cygan, R.T. (2016) Introduction to a special issue on molecular computer simulations of clays and clay-water interfaces: Recent progress, challenges, and opportunities. Clays and Clay Minerals, 64, 335–336.
Lagaly, G. (1981) Characterization of clays by organic compounds. Clay Minerals, 16, 1–21.
Lagaly, G. (1982) Layer charge heterogeneity in vermiculites. Clays and Clay Minerals, 30, 215–222.
Lagaly, G., Ogawa, M., and Dekany, I. (2013) Clay mineralorganic interactions. Pp. 435–505 in: Handbook of Clay Science, 2nd Edition (F. Bergaya and G. Lagaly, editors). Elsevier, Amsterdam
Laird, D.A., Scott, A.D., and Fenton, T.E. (1989) Evaluation of the alkylammonium method of determining layer charge. Clays and Clay Minerals, 37, 41–46.
Lee, J.F., Mortland, M.M., Chiou, C.T., and Boyd, S.A. (1989) Shape-selective adsorption of aromatic molecules from water by tetramethylammonium-smectite. Journal of the Chemical Society, 85, 2953–2962.
Li, Z.H., Jiang, W.T., and Hong, H.L. (2008) An FTIR investigation of hexadecyltrimethylammonium intercalation into rectorite. Spectrochimica Acta Part A, 71, 1525–1534.
Liu, X.D., Lu, X.C., Wang, R.C., Zhou, H.Q., and Xu, S.J. (2007) Interlayer structure and dynamics of alkylammonium- intercalated smectites with and without water: A molecular dynamics study. Clays and Clay Minerals, 55, 554–564.
Liu, X.D., Lu, X.C., Wang, R.C., Zhou, H.Q., and Xu, S.J. (2009) Molecular dynamics insight into the cointercalation of hexadecyltrimethyl-ammonium and acetate ions into smectites. American Mineralogist, 94, 143–150.
Loewenstein, W. (1954) The distribution of aluminum in the tetrahedra of silicates and aluminates. American Mineralogist, 39, 92–96.
Madejová, J., Sekeráková, L’., Bizovská, V., Slaný, M., and Jankovič, L’. (2016) Near-infrared spectroscopy as an effective tool for monitoring the conformation of alkylammonium surfactants in montmorillonite interlayers. Vibrational Spectroscopy, 84, 44–52.
Mykola, S., Olga, N., and Dmitry, M. (2016) The influence of alkylammonium modified clays on the fungal resistance and biodeterioration of epoxy-clay nanocomposites. International Biodeterioration & Biodegradation, 110, 136–140.
Nguemtchouin, M.G.M., Ngassoum, M.B., Kamga, R., Deabate, S., Lagerge, S., Gastaldi, E., Chalier, P., and Cretin, M. (2015) Characterization of inorganic and organic clay modified materials: An approach for adsorption of an insecticidal terpenic compound. Applied Clay Science, 104, 110–118.
Osman, M.A., Seyfang, G., and Suter, U.W. (2000) Twodimensional melting of alkane monolayers ionically bonded to mica. Journal of Physical Chemistry B, 104, 4433–4439.
Osman, M.A., Ernst, M., Meier, B.H., and Suter, U.W. (2002) Structure and molecular dynamics of alkane monolayers self-assembled on mica platelets. Journal of Physical Chemistry B, 106, 653–662.
Osman, M.A., Ploetze, M., and Skrabal, P. (2004) Structure and properties of alkylammonium monolayers self-assembled on montmorillonite platelets. Journal of Physical Chemistry B, 108, 2580–2588.
Pazos, M.C., Cota, A., Osuna, F.J., Pavon, E., and Alba, M.D. (2015) Self-assembling of tetradecylammonium chain on swelling high charge micas (Na-Mica-3 and Na-Mica-2): Effect of alkylammonium concentration and mica layer charge. Langmuir, 31, 4394–4401.
Perry, T.D., Cygan, R.T., and Mitchell, R. (2006) Molecular models of alginic acid: Interactions with calcium ions and calcite surfaces. Geochimica et Cosmochimica Acta, 70, 3508–3532.
Sarkar, M., Dana, K., and Ghatak, S. (2011) Evolution of molecular structure and conformation of n-alkylammonium intercalated iron rich bentonites. Journal of Molecular Structure, 1005, 161–166.
Scholtzová, E., Madejová, J., Jankovič, L., and Tunega, D. (2016) Structural and spectroscopic characterization of montmorillonite intercalated with n-butylammonium cations (N=1-4)-modeling and experimental study. Clays and Clay Minerals, 64, 401–412.
Smith, W. and Forester, T.R. (1996) DL_POLY_2.0: A general-purpose parallel molecular dynamics simulation package. Journal of Molecular Graphics, 14, 136–141.
Szczerba, M., Kuligiewicz, A., Derkowski, A., Gionis, V., Chryssikos, G.D., and Kalinichev, A.G. (2016) Structure and dynamics of water-smectite interfaces: Hydrogen bonding and the origin of the sharp O-Dw/O-Hw infrared band from molecular simulations. Clays and Clay Minerals, 64, 452–471.
Tambach, T.J., Boek, E.S., and Smit, B. (2006) Molecular order and disorder of surfactants in clay nanocomposites. Physical Chemistry Chemical Physics, 8, 2700–2702.
Vaia, R.A., Teukolsky, R.K., and Giannelis, E.P. (1994) Interlayer structure and molecular environment of alkylammonium layered silicates. Chemistry of Materials, 6, 1017–1022.
Wang, L.Q., Liu J., Exarhos, G.J., Flanigan, K.Y., and Bordia, R. (2000) Conformation heterogeneity and mobility of surfactant molecules in intercalated clay minerals studied by solid-state NMR. Journal of Physical Chemistry B, 104, 2810–2816.
Weiss, A. (1963) Organic derivatives of mica-type layer silicates. Angewandte Chemie International Edition, 2, 134–144.
Wen, X.Y., He, H.P., Zhu, J.X., Jun, Y., Ye, C.H., and Deng, F. (2006) Arrangement, conformation, and mobility of surfactant molecules intercalated in montmorillonite prepared at different pillaring reagent concentrations as studied by solid-state NMR spectroscopy. Journal of Colloid and Interface Science, 299, 754–760.
Zeng, Q.H., Yu, A.B., Lu, G.Q., and Standish, R.K. (2003) Molecular dynamics simulation of organic-inorganic nanocomposites: the layering behavior and interlayer structure of organoclays. Chemistry of Materials, 15, 4732–4738.
Zeng, Q.H., Yu, A.B., Lu, G.Q., and Standish, R.K. (2004) Molecular dynamics simulation of the structural and dynamic properties of organoclay. Journal of Physical Chemistry B, 108, 10025–10033.
Zhao, Q. and Burns, S.E. (2012) Molecular dynamics simulation of secondary sorption behavior of montmorillonite modified by single chain quaternary ammonium cations. Environmental Science & Technology, 46, 3999–4007.
Zhu, J.X., He, H.P., Zhu, L.Z., Wen, X.Y., and Deng, F. (2005) Characterization of organic phases in the interlayer of montmorillonite using FTIR and C-13 NMR. Journal of Colloid and Interface Science, 286, 239–244.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Liu, X., Zhang, Y. et al. Molecular Dynamics Simulation of Alkylammonium-Intercalated Vermiculites. Clays Clay Miner. 65, 378–386 (2017). https://doi.org/10.1346/CCMN.2017.064070
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2017.064070