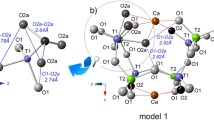
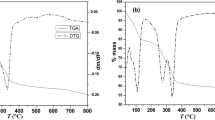
Abstract
Some aspects of the crystal structure of illite are not understood properly yet, in spite of its abundance and significance as a component of soils, sediments, and low-grade metamorphic rocks. The present study aimed to explore the role of hydronium cations in the interlayer space of illite in a theoreticalexperimental approach in order to clarify previous controversial reports. The infrared spectroscopy of this mineral has been studied experimentally and by means of atomistic calculations at the quantum mechanical level. The tetrahedral charge is critical for the stability of the hydronium cations, the presence of which has probably been underestimated in previous studies. In the present study, computational studies have shown that the hydronium cations in aqueous solutions are likely to be intercalated in the interlayer space of illite, exchanging for K cations. During the drying process these cations are stabilized by hydrogen bonds in the interlayer space of illite.
Similar content being viewed by others
References
Accelrys (2009) Accelrys Inc. Materials Studio, San Diego, California, USA.
Baer, M., Marx, D., and Mathias, G. (2011) Assigning predissociation infrared spectra of microsolvated hydronium cations H3O+(H2O)n (n = 0, 1, 2, 3) by ab initio molecular dynamics. ChemPhysChem, 12, 1906–1915.
Benco, L. and Tunega, D. (2009) Adsorption of H2O, NH3 and C6H6 on alkali metal cations in internal surface of mordenite and in external surface of smectite: a DFT study. Physics and Chemistry of Minerals 36, 281–290.
Bishop, J.L., Pieters, C.M., and Edwards, J.O. (1994) Infrared spectroscopic analyses on the nature of water in montmorillonite. Clays and Clay Minerals 42, 702–716.
Boulet, P., Greenwell, H.C., Stackhouse, S., and Coveney, P.V. (2006) Recent advances in understanding the structure and reactivity of clays using electronic structure calculations. Journal of Molecular Structure: Theochem, 762, 33–48.
Brorsen, K.R., Pruitt, S.R., and Gordon, M.S. (2014) Surface affinity of the hydronium Ion: The effective fragment potential and umbrella sampling. Journal of Physical Chemistry B, 118, 14382–14387.
Brown, G. and Norrish, K. (1952) Hydrous micas. Mineralogical Magazine, 29, 929–932.
Demontis, P., Masia, M., and Suffritti, G.B. (2013) Water nanoconfined in clays: the structure of Na vermiculite revisited by ab initio simulations. Journal of Physical Chemistry C, 117, 15583–15592.
Drits, V.A., Plançon, A., Sakharov, B.A., Besson, G., Tsipurski, S.I., and Tchoubar, C. (1984) Diffraction effects calculated for structural models of K-saturated montmorillonite containing different types of defects. Clay Minerals, 19, 541–561.
Escamilla-Roa, E. and Moreno, F. (2012) Adsorption of glycine by cometary dust: Astrobiological implications. Planetary and Space Science, 70, 1–9.
Escamilla-Roa, E. and Moreno, F. (2013) Adsorption of glycine on cometary dust grains: II — Effect of amorphous water ice. Planetary and Space Science 75, 1–10.
Escamilla-Roa, E. and Sainz-Díaz, C.I. (2014) Effect of amorphous ammonia-water ice onto adsorption of glycine on cometary dust grain and IR spectroscopy. Journal of Physical Chemistry C, 118, 26080–26090.
Escamilla-Roa, E., Hernández-Laguna, A., and Sainz-Díaz, C.I. (2013) Cation arrangement in the octahedral and tetrahedral sheets of cis-vacant polymorph of dioctahedral 2:1 phyllosilicates by quantum mechanical calculations. American Mineralogist, 98, 724–735.
Escamilla-Roa, E., Hernández-Laguna, A., and Sainz-Díaz, C.I. (2014) Theoretical study of the hydrogen bonding and infrared spectroscopy in the cis-vacant polymorph of dioctahedral 2:1 phyllosilicates. Journal of Molecular Modeling, 20, 1–15.
Fialips, C.I., Huo, D., Yan, L., Wu, J., and Stucki, J.W. (2002) Infrared study of seduced and reduced-reoxidized ferruginous smectite. Clays and Clay Minerals 50, 455–469.
Giese, R. (1979) Hydroxyl orientations in 2:1 phyllosilicates. Clays and Clay Minererals 27, 213–223.
Hower, J. and Mowatt, T.C. (1966) The mineralogy of illites and mixed-layer illite/montmoril lonites. American Mineralogist, 51, 825–854.
Kuligiewicz, A., Derkowski, A., Szczerba, M., Gionis, V., and Chryssikos, G.D. (2015) Revisiting the infrared spectrum of the water-smectite interface. Clays and Clay Minerals, 63, 15–29.
Leydier, F., Chizallet, C., Costa, D., and Raybaud, P. (2015) Revisiting carbenium chemistry on amorphous silica-alumina: Unraveling their milder acidity as compared to zeolites. Journal of Catalysis, 325, 35–47.
Liu, X., Lu, X., Sprik, M., Cheng, J., Meijer, E.J., and Wang, R. (2013) Acidity of edge surface sites of montmorillonite and kaolinite. Geochimica et Cosmochimica Acta, 117, 180–190.
Michot, L.J., Ferrage, E., Jiménez-Ruiz, M., Boehm, M., and Delville, A. (2012) Anisotropic features of water and ion dynamics in synthetic Na- and Ca-smectites with tetrahedral layer charge. A combined quasielastic neutron-scattering and molecular dynamics simulations study. Journal of Physical Chemistry C, 116, 16619–16633.
Morrow, C.P., Yazaydin, A.O., Krishnan, M., Bowers, G.M., Kalinichev, A.G., and Kirkpatrick, R.J. (2013) Structure, energetics, and dynamics of smectite clay interlayer hydration: Molecular dynamics and metadynamics investigation of Na-hectorite. Journal of Physical Chemistry C, 117, 5172–5187.
Nieto, F., Mellini, M., and Abad, I. (2010) The role of H3O+ in the crystal structure of illite. Clays and Clay Minerals, 58, 238–246.
Ortega-Castro, J., Hernández-Haro, N., Hernández-Laguna, A., and Sainz-Díaz, C.I. (2008) DFT calculation of crystallographic properties of dioctahedral 2:1 phyllosilicates. Clay Minerals, 43, 351–361.
Ortega-Castro, J., Hernández-Haro, N., Muñoz-Santiburcio, D., Hernández-Laguna, A., and Sainz-Díaz, C.I. (2009) Crystal structure and hydroxyl group vibrational frequencies of phyllosilicates by DFT methods. Journal of Molecular Structure: Theochem, 912, 82–87.
Perdew, J.P., Burke, K., and Ernzerhof, M. (1996) Generalized gradient approximation made simple. Physics Review Letters, 77, 3865.
Rieder, M., Cavazzini, G., D’yakonov, Y.S., Frank-Kamenetskii, V.A., Gottardi, G., Guggenheim, S., Koval, P.V., Mueller, G., Neiva, A.M.R., Radoslovich, E.W., Robert, J.-L., Sassi, F.P., Takeda, H., Weiss, Z., and Wones, D.R. (1998) Nomenclature of the micas. Clays and Clay Minerals, 46, 586–595.
Russell, J. and Fraser, A. (1971) IR spectroscopic evidence for interaction between hydronium ions and lattice OH groups in montmorillonite. Clays and Clay Minerals, 19, 55–59.
Sainz-Díaz, C.I., Hernández-Laguna, A., and Dove, M.T. (2001) Theoretical modelling of cis-vacant and trans-vacant configurations in the octahedral sheet of illites and smectites. Physics and Chemistry of Minerals, 28, 322–331.
Sainz-Díaz, C.I., Palin, E.J., Dove, M.T., and Hernández-Laguna, A. (2003) Monte Carlo simulations of ordering of Al, Fe, and Mg cations in the octahedral sheet of smectites and illites. American Mineralogist, 88, 1033–1045.
Sainz-Díaz, C.I., Escamilla-Roa, E., and Hernández-Laguna, A. (2005) Quantum mechanical calculations of trans-vacant and cis-vacant polymorphism in dioctahedral 2:1 phyllosilicates. American Mineralogist, 90, 1827–1834.
Tokiwai, K. and Nakashima, S. (2010) Integral molar absorptivities of OH in muscovite at 20 to 650°C by in-situ high-temperature IR microspectroscopy. American Mineralogist, 95, 1052–1059.
Wang, J., Kalinichev, A.G., Kirkpatrick, R.J., and Cygan, R.T. (2005) Structure, energetics, and dynamics of water adsorbed on the muscovite (001) surface: A molecular dynamics simulation. Journal of Physical Chemistry B, 109, 15893–15905.
White, J.L. and Burns, A.F. (1963) Infrared spectra of hydronium ion in micaceous minerals. Science, 141, 800–801.
Xu, W., Johnston, C.T., Parker, P., and Agnew, S.F. (2000) Infrared study of water sorption on Na, Li, Ca, and Mg-exchanged (SWy-1 and SAz-1) montmorillonite. Clays and Clay Minerals, 48, 120–131.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escamilla-Roa, E., Nieto, F. & Sainz-Díaz, C.I. Stability of the Hydronium Cation in the Structure of Illite. Clays Clay Miner. 64, 413–424 (2016). https://doi.org/10.1346/CCMN.2016.0640406
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2016.0640406