Abstract
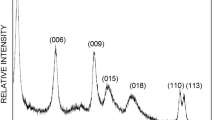
A rhamnolipid-layered double hydroxide (RL-LDH) nanocomposite, derived from the rhamnolipid (RL) biosurfactant, was synthesized through a delamination/reassembling process. The adsorption characteristics of Cu(II) on RL-LDH were investigated in detail and the results indicated the potential of using RL-LDH as an environmentally friendly adsorbent to remove Cu(II). The fabricated RL-LDH nanocomposite was characterized using powder X-ray diffraction, Fourier-transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, elemental chemical composition, and specific surface area analyses. Batch adsorption experiments were conducted to study the influence of various factors, such as contact time, initial Cu(II) concentration, temperature, initial solution pH, and electrolyte concentration on Cu(II) adsorption by the RL-LDH nanocomposite. The RL-LDH nanocomposite had a low surface area of 11.71 m2 g−1, which suggests that surface adsorption would not be important in Cu(II) adsorption. The Cu(II) adsorption data fitted the Freundlich model well at pH 5.5, whereas the adsorption kinetics were accurately described by a pseudo-second-order kinetics model. Chemical binding, that is, the formation of a RL-Cu(II) complex in the LDH interlayer, was assumed to be the rate-limiting step in the adsorption process. Thermodynamic parameters that included Gibbs free energy, enthalpy, and entropy changes were also calculated. The adsorption was found to be a spontaneous and exothermic chemisorption process. Furthermore, the adsorption properties of RL-LDH for Cu(II) were compared to Cu(II) adsorption using other adsorbents.
Similar content being viewed by others
References
Anirudhan, T.S., Jalajamony, S., and Suchithra, P.S. (2009) Improved performance of a cellulose-based anion exchanger with tertiary amine functionality for the adsorption of chromium (VI) from aqueous solutions. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 335, 107–113.
Awual, M.R., Ismael, M., Yaita, T., El-Safty, S.A., Hideaki, S., Okamoto, Y., and Suzuki, S. (2013) Trace copper(II) ions detection and removal from water using novel ligand modified composite adsorbent. Chemical Engineering Journal, 222, 67–76.
Bai, G., Brusseau, M.L., and Miller, R.M. (1997) Biosurfactant-enhanced removal of residual hydrocarbon from soil. Applied and Environmental Microbiology, 63, 157–170.
Bhattacharyya, K.G. and Gupta, S.S. (2011) Removal of Cu(II) by natural and acid-activated clays: An insight of adsorption isotherm, kinetic and thermodynamics. Desalination, 272, 66–75.
Bosso, S.T. and Enzweiler, J. (2002) Evaluation of heavy metal removal from aqueous solution onto scolecite. Water Research, 36, 4795–4800.
Chuang, Y.H., Liu, C.H., Tzou, Y.M., Chang, J.S., Chiang, P.N., and Wang, M.K. (2010) Comparison and characterization of chemical surfactants and bio-surfactants intercalated with layered double hydroxides (LDHs) for removing naphthalene from contaminated aqueous solutions. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 366, 170–177.
da Fonseca, M.G., de Oliveira, M.M., Arakaki, L.N.H., Espinola, J.G.P., and Airoldi, C. (2005) Natural vermiculite as an exchanger support for heavy cations in aqueous solution. Journal of Colloid and Interface Science, 285, 50–55.
Dahrazma, B. and Mulligan, C.N. (2007) Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemosphere, 69, 705–711.
Dubinin, M.M. and Radushkevich, L.V. (1947) Equation of the characteristic curve of activated charcoal. Proceedings of the Academy of Sciences of the USSR, Physical Chemistry Section, 55, 331–333.
Freundlich, H.M.F. (1906) Over the adsorption in solution. Journal of Physical Chemistry, 57, 385–470.
Gosset, T., Trancart, J.L., and Thevenot, D.R. (1986) Batch metal removal by peat kinetics and thermodynamics. Water Research, 20, 21–26.
Hatay, I., Gup, R., and Ersz, M. (2008) Silica gel functionalized with 4-phenylacetophynone 4-aminobenzoylhydrazone: synthesis of a new chelating matrix and its application as metal ion collector. Journal of Hazardous Materials, 150, 546–553.
Huang, G., Wang, D., Ma, S., Chen, J., Jiang, L., and Wang, P. (2015) A new, low-cost adsorbent: Preparation, characterization, and adsorption behavior of Pb(II) and Cu(II). Journal of Colloid and Interface Science, 445, 294–302.
Inyang, M., Gao, B., Yao, Y., Xue, Y., Zimmerman, A.R., Pullammanappallil, P., and Cao, X. (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresource Technology, 110, 50–56.
Kameda, T., Saito, S., and Umetsu Y. (2005) Mg-Al layered double hydroxide intercalated with ethylene-diaminetetraacetate anion: Synthesis and application to the uptake of heavy metal ions from an aqueous solution. Separation and Purification Technology, 47, 20–26.
Koyuncu, H. and Kul, A.R. (2014) An investigation of Cu(II) adsorption by native and activated bentonite: Kinetic, equilibrium and thermodynamic study. Journal of Environmental Chemical Engineering, 2, 1722–1730.
Lagergren, S. (1898) Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar, 24, 1–39.
Langmuir, I. (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American Chemical Society, 38, 2221–2295
Lu, X., Meng, L., Li, H., Du, N., Zhang, R., and Hou, W. (2013) Facile fabrication of ibuprofen-LDH nanohybrids via a delamination/reassembling process. Materials Research Bulletin, 48, 1512–1517.
Matlock, M.M., Howerton, B.S., and Atwood, D.A. (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Research, 36, 4757–4764.
Miyata, S. (1983) Anion-exchange properties of hydrotalcite-like compounds. Clays and Clay Minerals, 31, 305–311.
Mohammadi, T., Moheb, A., Sadrzadeh, M., and Razmi, A. (2005) Modeling of metal ion removal from wastewater by electrodialysis. Separation and Purification Technology, 41, 73–82.
Mulligan, C.N. (2009) Recent advances in the environmental applications of biosurfactants. Current Opinion in Colloid and Interface Science, 14, 372–378.
Ochoa-Loza, F.J., Artiola, J.F., and Maier, R.M. (2001) Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. Journal of Environmental Quality, 30, 479–485.
Onyango, M.S., Kojima, Y., Kumar, A., and Kuchar, D. (2006) Uptake of fluoride by Al3+ pretreated low-silica synthetic zeolites: adsorption equilibrium and rate studies. Separation and Purification Technology, 41, 683–704.
Qiu, D. and Hou, W. (2009) Synthesis and characterization of indole-3-butyric acid/hydrotalcite-like compound nanohybrids. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 336, 12–17.
Ruan, X., Huang, S., Chen, H., and Qian, G. (2013) Sorption of aqueous organic contaminants onto dodecyl sulfate intercalated magnesium iron layered double hydroxide. Applied Clay Science, 72, 96–103.
Sağ, Y. and Aktay, Y. (2001) Mass transfer and equilibrium studies for the sorption of chromium ions onto chitin. Process Biochemistry, 36, 157–173.
Shan, R.R., Yan, L.G., Yang, K., Yu, S.J., Hao, Y.F., Yu, H.Q., and Du B. (2014) Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution. Chemical Engineering Journal, 252, 38–46.
Sheng, G.D., Li, J.X., Shao, D.D., Hu, J., Chen, C.L., Chen, Y.X., and Wang, X.K. (2010) Adsorption of copper(II) on multiwalled carbon nanotubes in the absence and presence of humic or fulvic acids. Journal of Hazardous Materials, 178, 333–340.
Sud, D., Mahajan, G., and Kaur, M.P. (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions — a review. Bioresource Technology, 99, 6017–6027.
Torrens, J.L., Herman, D.C., and Maier, R.M. (1998) Biosurfactant (rhamnolipid) sorption and the impact on rhamnolipid-facilitated removal of cadmium from various soils under saturated flow conditions. Environmental Science & Technology, 32, 776–781.
Venkateswaran, P., Gopalakrishnan, A.N., and Palanivelu, K. (2007) Di(2-ethylhexyl)phosphoric acid-coconut oil supported liquid membrane for the separation of copper ions from copper plating wastewater. Journal of Environmental Sciences, 19, 1446–1453.
Wan Ngah, W.S., Teong, L.C., Toh, R.H., and Hanafiah, M.A.K.M. (2013) Comparative study on adsorption and desorption of Cu(II) ions by three types of chitosan-zeolite composites. Chemical Engineering Journal, 223, 231–238.
Wang, J., Zhou, J., Li, Z., Song, Y., Liu, Q., Jiang, Z., and Zhang, M. (2010) Magnetic, luminescent Eu-doped Mg-Al layered double hydroxide and its intercalation for ibuprofen. Chemistry — A European Journal, 16, 14404–14411.
Wu, Q., Olafsen, A., Vistad, Ø.B., Roots, J., and Norby, P. (2005) Delamination and restacking of a layered double hydroxide with nitrate as counter anion. Journal of Materials Chemistry, 15, 4695–4700.
Zang, Y.B., Hou, W.G., and Xu, J. (2011) Removal of Cu(II) from CuSO4 aqueous solution by Mg-Al hydrotalcite-like compounds. Chinese Journal of Chemistry, 29, 847–852.
Zhang, Y., Chen, Y., Wang, C., and Wei, Y. (2014) Immobilization of 5-aminopyridine-2-tetrazole on cross-linked polystyrene for the preparation of a new adsorbent to remove heavy metal ions from aqueous solution. Journal of Hazardous Materials, 276, 129–137.
Zhao, Y.G., Shen, H.Y., Pan, S.D., Hu, M.Q., and Xia, Q.H. (2010a) Preparation and characterization of amino-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium(VI) ions. Journal of Materials Science, 45, 5291–5301.
Zhao, G., Zhang, H., Fan, Q., Ren, X., Li, J., Chen, Y., and Wang, X. (2010b) Sorption of copper(II) onto super-adsorbent of bentonite-polyacrylamide composites. Journal of Hazardous Materials, 173, 661–668.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Bi, HY., Li, H. et al. Adsorption of Cu(II) on Rhamnolipid-Layered Double Hydroxide Nanocomposite. Clays Clay Miner. 64, 560–570 (2016). https://doi.org/10.1346/CCMN.2016.064034
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.2016.064034