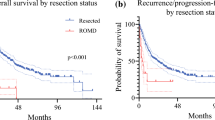
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is most often metastatic at diagnosis. As systemic therapy continues to improve alongside advanced surgical techniques, the focus has shifted toward defining biologic, rather than technical, resectability. Several centers have reported metastasectomy for oligometastatic PDAC, yet the indications and potential benefits remain unclear. In this review, we attempt to define oligometastatic disease in PDAC and to explore the rationale for metastasectomy. We evaluate the existing evidence for metastasectomy in liver, peritoneum, and lung individually, assessing the safety and oncologic outcomes for each. Furthermore, we explore contemporary biomarkers of biological resectability in oligometastatic PDAC, including radiographic findings, biochemical markers (such as CA 19-9 and CEA), inflammatory markers (including neutrophil-to-lymphocyte ratio, C-reactive protein, and scoring indices), and liquid biopsy techniques. With careful consideration of existing data, we explore the concept of biologic resectability in guiding patient selection for metastasectomy in PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy, which by 2030 will be the second-leading cause of cancer-related deaths in the United States.1 Despite advances in systemic therapy and surgical techniques, most patients present with unresectable metastatic disease. Twenty-five percent of patients are considered “borderline resectable” or “locally advanced” as defined by National Comprehensive Cancer Network (NCCN) and National Cancer Institute (NCI) treatment guidelines.2
In recent years, advances in the combination therapies FOLFIRINOX and gemcitabine-based perioperative treatment have increased overall survival (OS) in the metastatic setting, and these trials have impacted the treatment paradigm in borderline resectable disease.3,4,5 Increased utilization of multiagent chemotherapy has opened up possibilities for surgical intervention in cases that were once thought to be unresectable. In many cases, the discussion has shifted from one of “technical” resectability to those of “biologic” resectability.6,7
Growing literature suggests that clinicopathologic risk factors and tumor biology may impact the pattern of disease and site of the first recurrence. Sites of recurrence after curative-intent resection include liver (25.6%), locoregional (20.8%), peritoneal dissemination (13.5%), and lung (11.4%); additionally, many cases may present with multisite recurrence.8 Studies comparing site of metastatic disease have demonstrated differences that suggest multiple PDAC phenotypes, each associated with a different type of tumor biology and behavior.9
While historically, the presence of metastases in PDAC has been a contraindication for curative-intent resection, population and observational studies in the past decade have reported technical feasibility and safety of metastasectomy for oligometastatic disease. Metastasectomy has already been well-documented in colon cancer, melanoma, renal cell carcinoma, and certain types of sarcoma.10,11,12,13 Although there are several studies on metastasectomy in PDAC, its adoption is still controversial, and evidence is not yet strong enough to influence any guidelines. The wide variation in tumor biology, response to therapy, and recurrence raises the question: Are there certain biologic and patient-specific factors that may dictate oncologic success of metastasectomy?
In this paper, we will attempt to define oligometastatic disease in PDAC, review recent literature on metastasectomy to clarify safety and oncologic outcomes, and finally, highlight the nuances of patient and tumor characteristics to explore the potential role for surgical resection of oligometastatic pancreatic cancer.
Defining Oligometastatic Disease
The term “oligometastasis” was first described in 1995 by Hellman and Weichselbaum, who theorized that early in the progression of a malignancy, a limited number of metastases may appear, before to the development of exponential metastatic growth.14 Since then, several others have expanded upon or differed from this hypothesis. A study by Lussier et al. comparing microRNA expression of tumor samples from oligometastatic patients found that some of these patients failed to progress to polymetastases; these samples were characterized by distinct microRNA features.15 In colorectal cancer, several studies have identified specific gene mutations, such as ERBB2, and regression of key-driver gene mutations (KRAS, PIK3CA) that are associated with oligometastatic clinical behavior.11,16,17 These findings suggest that oligometastatic disease may be a distinct entity from polymetastatic disease, rather than an earlier timepoint in the inevitable timeline of metastatic spread.
Use of the term “oligometastasis” has increased in recent years; however, its definitions and implications have remained nebulous. A recent metanalysis by Rim et al. investigating the role of local consolidative therapy for oligometastasis found that 48.1% of studies defined oligometastasis as up to five lesions, 7.4% up to four lesions, and 25.9% as up to three lesions.18 These cutoffs are seemingly arbitrary, and this lack of definition also is reflected in clinical practice. A survey of medical and radiation oncologists found no common understanding of oligometastatic disease and significant variability in treatment recommendations.19
A recent ASTRO/ESTRO (American Society for Radiation Oncology/European Society for Radiotherapy and Oncology) consensus study proposed a definition for oligometastatic disease that centered on the ability to deliver safe and meaningful radiotherapy with curative intent to all metastatic sites.20 Perhaps, then, the surgeon should define oligometastatic disease as that which can be safely operated on with a reasonable chance of cure or significant prolongation of life. The reality is that the clinical state of “oligometastasis” is poorly understood. To date, the decision of whether to operate on these patients is limited by surgeon and institute experience and individual patient preference.
Rationale for Metastasectomy in Pancreatic Ductal Adenocarcinoma
Metastasectomy may be justified when it is safe and offers a survival benefit, improved quality of life, or a possibility of cure. Metastasectomy for patients with stage IV colon cancer now offers a potential for cure, and liver metastasectomy in carefully selected patients provides a 40–60% chance of 5 year survival.16,21 In patients with pancreatic neuroendocrine tumors (PNETs) undergoing liver-directed therapy, the 5 year overall survival has been reported as up to 80%.22 In the case of metastatic melanoma, BRAF inhibitors, and CTLA-4 and PD-1 inhibitors have been shown to induce a rapid response and conversion to oligometastatic disease, creating the opportunity for metastasectomy and resulting in 40% 5 year survival in patients with stage IV disease.23,24 These studies demonstrate the importance of systemic control to allow for metastasectomy.
It has been observed that there are subtypes of metastatic PDAC that may respond differently to the same treatment. This is in part because of complex genomic rearrangements and nonconventional mutagenesis of key driver genes (KRAS, TP53, SMAD4, and CDKN2A) resulting in rapid tumor progression.25 These genetic and molecular differences may lead to differences in tissue tropism, and subsequently, differences in response to therapy and patient outcomes. In the following sections, we will separately approach liver, lung, and peritoneum as sites of metastatic PDAC, and review the existing data surrounding metastasectomy.
Liver Metastases in PDAC
The liver is the most common organ for initial metastatic spread or distant recurrence in patients with pancreatic cancer.8 Of patients presenting with metastatic PDAC, 87.7% have synchronous liver metastases. Additionally, despite advances in imaging technology, 8% of patients may have occult metastatic disease at the time of surgical exploration.26 Furthermore, in patients with limited local PDAC who have undergone resection, 26.5% later develop metachronous liver disease.27
This liver tropism may be explained by the portal venous blood supply and lymphatic drainage, which provide means for hematogenous and lymphatic spread, respectively. There also is evidence that genetic alterations in TP53 and TGF-beta signaling might predict the pattern of metastatic progression.28 Interestingly, after metastasectomy, the recurrence of metastatic disease occurs in the same organ in most cases, supporting the concept of molecular programing for metastatic disease.29
Role of Preoperative Chemotherapy in Downstaging PDAC Liver Metastases
Systemic therapies, such as FOLFIRINOX and gemcitabine + nab-paclitaxel, have improved clinical outcomes and survival in patients with metastatic PDAC (mPDAC).30,31 Frigerio and colleagues performed a prospective study of mPDAC patients, in which 24 patients with mPDAC had complete radiologic disappearance of liver metastasis after preoperative chemotherapy and underwent curative-intent resection of the primary tumor. Eighty-eight percent of these patients had an R0 resection of the primary tumor, and the median disease-free survival was 21 months after diagnosis.32 Despite the small sample size, this study shows the potential value of preoperative chemotherapy in controlling metastatic disease and rendering the tumor operable.
Nagai et al. investigated PDAC patients undergoing liver resection for isolated metastasis to identify favorable factors associated with survival. The overall survival in patients who received preoperative chemotherapy followed by surgery was 24 months from surgery compared with only 10.6 months in patients who underwent just “upfront” surgery (p = 0.01).33 These findings demonstrate the importance of systemic treatment in conjunction with operative intervention to help to identify patients with more favorable tumor biology.
Despite several studies evaluating liver metastases, the median survival for patients with liver metastases from PDAC has remained poor compared with other sites of metastases, regardless of treatment approach.34 In a study by Groot et al. reviewing PDAC patients who experienced recurrence after pancreatectomy, patients with multiple-site recurrence (4.7 months) or liver-only recurrence (7.2 months) had significantly worse median survival compared with lung-only recurrence (15.5 months) or local-only recurrence (9.7 months).35 In the absence of precise predictors of biologic behavior, all patients with metastatic PDAC should be evaluated for receipt of systemic therapy before consideration of liver resection.
Surgical Outcomes for Synchronous and Metachronous Liver Resections
During the past decade, several studies have published surgical outcomes for patients undergoing liver resection for synchronous or metachronous metastatic lesions in PDAC (Table 1). Bachellier et al. reviewed 92 patients who underwent resection of PDAC with synchronous liver metastases.36 A variety of operations were undertaken, including pancreaticoduodenectomy (53.2%), total pancreatectomy (5.4%), and distal pancreatectomy with splenectomy (41.3%). Venous and arterial resections were performed in 4% and 8% of patients, respectively, and 18.4% of patients had associated visceral resections (stomach and left colon). With regards to liver metastases, 50% of patients had only a single liver lesion, and liver resections were mostly nonanatomic or minor resections (67%), RFA only (20.6%), or resection and RFA (11.9%), with one patient undergoing left hepatectomy. The overall 90 day morbidity and mortality rates in the entire cohort were 40.2% and 5.4%, respectively. The only difference in postoperative outcomes was higher postoperative pancreatic fistula in left pancreatic resection versus right pancreatic resection (18% vs. 0%). Given the variability in operative approach and differences in extent of resection seen across publications (Table 1), it is difficult to establish broad guidelines for liver resection in PDAC with respect to surgical outcomes.
There also have been studies examining the management of metachronous liver lesions from PDAC. In a retrospective review of 128 patients, Hackert et al. evaluated patients undergoing primary tumor and metastasis resection for PDAC and found a postoperative morbidity and mortality of 45% and 2.9%, respectively, for patients undergoing synchronous resection, and for patients undergoing liver resection only for metachronous metastases, it was 21.7% and 4.3%, respectively.41 The majority of patients with liver metastases (86%) had nonanatomic resections of one to four lesions. Only 14% of patients received formal resections, including bisegmentectomies and right/extended right hepatectomies.
Another multicenter study by Schwarz et al. identified patients who underwent hepatectomy for metachronous PDAC liver metastases to assess postoperative outcomes and overall survival.44 The median number of metastases in the group was 1, and overall postoperative morbidity was found to be 32%, which is comparable to existing data regarding postoperative morbidity after liver resection.
The majority of these studies have been performed in experienced centers by surgeons specializing in hepatobiliary operations. Although these data show acceptable postoperative morbidity and mortality, the feasibility and safety of liver resection for PDAC metastases should be evaluated in the context of metastatic tumor burden and surgeon and center experience.
Oncological Outcomes in Patients Undergoing Resection for Synchronous Liver Metastases
Current studies on liver metastasectomy in PDAC have reported a variety of oncologic outcomes, although sample sizes are small, and there are discrepancies in reported outcome variables (Table 2).
In a small case-control study, Kandel et al. reported a median OS of 2.7 years in patients (n = 6) who underwent preoperative chemotherapy and synchronous hepatic resection, R0/R1 primary tumor resection, and adjuvant therapy. 45 The median OS in this cohort was similar that of PDAC patients without metastatic disease (n = 8) who underwent curative intent resection ± preoperative chemotherapy (OS 2.02 years) and was superior to the median OS (0.98 years) in patients with metastatic disease (n = 18), who received chemotherapy only. Shao et al. found that overall survival in patients who had curative-intent resection with liver metastasectomy had an improved survival (16 months) compared with a matched control group of patients who had palliative surgery only (6 months).38
As mentioned previously, Hackert and colleagues presented one of the largest series on liver metastasectomy (n = 128) in patients with isolated liver oligometastatic disease (with 1–3 liver metastases). However, only 20 patients (15.6%) received preoperative chemotherapy, and only 57% received postoperative adjuvant chemotherapy (gemcitabine being the most commonly administered, 79.5%). Patients with synchronous resection had median survival of 10.6 months, and metachronous resection had a median survival of 14.8 months from liver resection.41
Frigerio et al. performed a retrospective analysis of 52 patients with liver-only synchronous metastasis, with 73.1% of patients having greater than two liver metastases.46 All patients received preoperative chemotherapy (63.5% FOLFIRINOX, 36.5% gemcitabine-based) and had complete regression of metastatic lesions before surgical intervention on cross-sectional imaging. Of total patients, 67.3% had normalized Ca19-9 posttreatment. With an 86.5% R0 resection rate, the overall survival from diagnosis was 37.2 months, and median disease-free survival (DFS) after pancreatectomy was 16.5 months. Of total patients, 75% experienced recurrence, and multivariate analysis found omission of adjuvant therapy to be associated with recurrence. The improved overall survival in this series demonstrates the importance of chemotherapy to help select for favorable biology.
Some of these studies also have sought to identify factors associated with improved prognosis in attempts to guide patient selection. Frigerio et al. found that neutrophil-to-lymphocyte ratio <1.7 was significantly associated with improved overall survival and disease-free survival.46 Bachellier et al. identified that Ca19-9 <500 at diagnosis, R0 resection, and administration of adjuvant chemotherapy were independent prognostic factors for overall survival.36 These factors are further discussed in subsequent sections.
There are two ongoing clinical trials that will prospectively evaluate the potential benefit of surgery and the role of perioperative chemotherapy for oligometastatic PDAC to the liver. The first is the CSPAC-1 trial, opened in 2018 by the Chinese Study Group for Pancreatic Cancer.47 This is a phase 3 trial that will include 1000–1200 patients who meet inclusion criteria of: three or fewer lesions anywhere in the liver; a pathologic diagnosis of PDAC; and ECOG 0/1. After first-line chemotherapy, response will be assessed via RECIST criteria, and patients entering the second step of the trial will be randomized to simultaneous resection of primary pancreatic cancer and liver metastases, or standard chemotherapy. The primary endpoint is overall survival from the time of enrolment.
The second clinical trial, HOLIPANC, opened in Germany in 2021, is a single-arm phase 2 trial in which data will be collected from patients with oligometastatic PDAC getting chemotherapy with the NAPOX (liposomal irinotecan, oxaliplatin, 5-fluouracil, folinic acid) chemotherapy regimen, followed by R0/R1 resection.48 The results of these clinical trials help elucidate predictors of better biology and the potential role for resecting synchronous liver metastases.
Oncological Outcomes in Patients Undergoing Resection for Metachronous Liver Metastases
Studies have reported favorable oncological outcomes in patients undergoing multimodal therapy for metachronous liver metastasis. Schwarz et al. performed a retrospective multicenter study of 25 patients undergoing hepatectomy for metachronous PDAC liver metastases and found that the median OS was 36.8 months from diagnosis compared with 9.2 months in patients who received chemotherapy only.44 A study by Zanini et al. found that median OS was significantly higher in patients with metachronous metastases undergoing liver resection compared with synchronous metastases.49 These studies suggest that surgical intervention for metachronous metastases may be associated with improved survival for certain patients.
In a retrospective study, Mitsuka et al. showed that patients with solitary metachronous liver metastases who underwent liver resection had improved median survival (55 months) compared with those patients who did not undergo liver resection (17.5 months).50 In this study, surgical resection was offered to patients who had no evidence of disease progression based on CT imaging during a 3 month observational period and were considered for a second metastasectomy if disease-free interval (DFI) was >12 months from the first liver resection. These data suggest that as in other malignancies, the DFI may be a good predictor of patients that could benefit from surgical resection of oligometastases.
Peritoneal Metastases in PDAC
Peritoneal carcinomatosis (PC) is a hallmark of advanced-stage disease and historically has been associated with very poor outcomes, regardless of the primary tumor site. However, in patients with colorectal cancer (CRC), peritoneal carcinomatosis (PC) has been reclassified as locoregional disease, which drastically changed the surgical approach and allowed for use of cytoreductive surgery (CRS) with intraperitoneal chemotherapy (IPC).51,52,53
A prospective clinical trial by Yamada et al. investigated patients undergoing surgery for resectable PDAC, and if peritoneal dissemination or positive peritoneal cytology was encountered during staging surgery, intraperitoneal paclitaxel was administered.54 Of these 79 patients, 20.3% then underwent pancreatectomy for the primary tumor. However, after surgery, 75% of patients experienced recurrence. Such data suggest that in contrast to CRC, peritoneal carcinomatosis in PDAC may be far more aggressive and represent an advanced state of disease.
Despite these findings, there have been attempts at downstaging peritoneal disease in PDAC. Yamamoto and colleagues compared 43 patients receiving intraperitoneal chemotherapy (IPC) to 49 patients who received standard chemotherapy.55 The overall median survival time was longer in patients who underwent surgical resection than those who did not (27.4 months vs. 11.3 months). These data suggest that surgical interventions may provide meaningful extensions to overall survival after peritoneal metastases are downstaged via multimodal therapies.
Expanding on this principle, a newer technique, pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been utilized in patients with peritoneal diseases mainly for palliation in patients with PC from various primary cancers.56,57 While several studies have demonstrated safety, ongoing prospective clinical trials are needed to evaluate efficacy.
Lung Metastases in PDAC
The lung is a common site for metastases in patients with PDAC; however, unlike hepatic metastases, lung metastases are most frequently metachronous, presenting as a late recurrence of PDAC. Patients with pulmonary metastases have been shown to have a longer time between pancreatectomy and recurrence and also have better OS than those with other types of recurrence.58,59 Pulmonary metastasectomy (PM) has in recent years been recognized as a procedure for patients with PDAC with reported 5 year survival of 31.1–69.8%, although data are limited (Table 3).60,61,62,63
Several studies have evaluated oncologic outcomes in patients undergoing pulmonary metastasectomy. Yun et al. identified 83 patients in their study who, after pancreatectomy, developed metachronous pulmonary metastases (27.7% single metastasis, 34.9% oligometastases with 2–5 lesions, and 37.3% multiple metastases). In the entire study population, the 5 year OS was 60.6% in patients who underwent PM compared with 6.2% in patients who received only chemotherapy or supportive care; however, overall survival also was directly related to the number of metastases.65 These differences in the scale of improvement are likely explained by patient selection.
Several studies have proposed factors associated with improved survival after PM or lobectomy. Nakajima et al. evaluated multiple case reports and found that a disease-free interval (DFI) >20 months and size of lung metastases <1.6 cm were associated with longer survival after lobectomy.66 Other studies have investigated biologic factors. Homma et al. found that higher numbers of tumor-infiltrating lymphocytes in the lung metastases and CD8+ lymphocytes in the primary PDAC specimen were a favorable prognostic factor.59
At this time, cautiously proceeding with metastasectomy for metachronous pulmonary lesions from PDAC may be safe and efficacious in carefully selected patients. Futures studies are needed to better define a biologically distinct subgroup of patients in whom metastasectomy is indicated.
Prognostic Factors for Improved Outcomes After Metastasectomy
Radiographic Findings
Macroscopically, primary tumor size larger than 2–3 cm has been associated with worse outcomes in some studies, whereas R0 resection is a significant indicator of improved survival.42,67,68 More than five liver metastases also has found to be associated with worse survival.69,70 Reports on the significance of the location of liver metastasis (central vs. peripheral) and lung lesions are not conclusive, and the significance of these factors in predicting surgical and survival outcomes for patients with mPDAC is unknown.
Radiographic response, or tumor shrinkage, has been widely used as a surrogate of therapy response and tumor behavior, although there are currently no consensus guidelines on what should be used as a surgical indication for curative-intent resection. Frigerio and colleagues used the criteria of radiographic response in parallel with normalized CA 19-9 levels while receiving preoperative chemotherapy, although the majority of these patients recurred after surgery.32 A clinical response, as measured by Response Evaluation Criteria in Solid Tumors (RECIST), has been widely used in other tumors and is now gaining popularity in PDAC. In a review of 11 studies, Satoi et al. found that patients with unresectable PDAC with complete or partial response based on RECIST criteria, in parallel with CA 19-9 <150 U/ml following preoperative chemotherapy, had better OS after curative intent-resection than patients with stable disease.71
An alternative criterion, PET Response Criteria in Solid Tumors (PERCIST), also has been proposed.72 Additionally, Abdelrahaman et al. found that in patients with borderline or locally advanced PDAC undergoing preoperative chemotherapy followed by resection, metabolic response as measured by FDG-PET was the largest independent preoperative predictor of pathologic response, recurrence-free survival, and overall survival, even when taking into account biochemical markers, such as Ca19-9.73,74 Such radiographic criteria may be useful in determining response after initial downstaging treatment, although they have yet to be implemented in any prospective clinical trials.
Systemic Biomarkers
Currently, the only FDA-approved PDAC biomarker is CA 19-9, with a median sensitivity of 79% and specificity of 82%. Takeda et al. identified that patients with liver-only metastases had a median Ca19-9 of 2780 U/mL compared with 6361 U/mL in patients with multiorgan metastases. On multivariate analysis, it was found that patients with oligometastatic liver disease with a Ca19-9 <1000 U/mL had improved overall survival. Additionally, 13 patients undergoing surgery after receipt of preoperative chemotherapy had an improved overall survival of 54.6 months compared with 20.8 months in patients who did not undergo surgery. While the range of pretreatment Ca19-9 values in the surgery group was wide, ranging from 4 to 50,000 U/mL, all patients in this group had normalization of Ca19-9 to 36 U/mL or less after preoperative chemotherapy.75
Tanaka et al. found that after FOLFIRINOX therapy, a CA 19-9 delta score (CA 19-9 postchemotherapy—CA 19-9 prechemotherapy) of 870 U/ml had 48% sensitivity and 81% specificity in predicting successful liver metastasectomy and primary tumor resection.8 These data suggest that CA19-9 reduction after preoperative chemotherapy may help to guide the decision to pursue metastasectomy.
While CA 19-9 has the potential to be used as marker of tumor response, variations in the data and a lack of clear cutoff values compromises its generalizability. In the study done by Frigerio at al., patients with synchronous liver metastases whose Ca19-9 had normalized after perioperative chemotherapy were selected for resection, and this was found to correlate significantly with improved OS.32 However, despite specifying a Ca19-9 decrease >50% relative to baseline as a selection criteria for metastasectomy, within this group, neither Ca19-9 decrease, nor posttreatment normalization were independently associated with survival.46 Additionally, and perhaps more importantly, approximately 5–7% of the population, belonging to the Le(a−b−) blood group, are unable to express Ca19-9, which compromises its ability to be used as a universal solitary biochemical marker.76,77
Carcinoembryonic antigen (CEA) also has been proposed as a biomarker. Studies in patients with pancreatic cancer have demonstrated that an elevated CEA at diagnosis was associated with a poorer overall survival compared with patients with a normal serum CEA.78 In a study by Hank et al., 93 patients with oligometastatic disease underwent metastasectomy along with resection of the primary tumor. Of this group, 45 patients had complete response of metastases on review of final pathology.79 This group was found to have significantly lower blood CEA levels. Such findings suggest that future studies should investigate the role of elevated CEA as a negative prognostic factor when deciding whether or not to perform metastasectomy.
Inflammatory Markers
Recently, the concept of systemic inflammation in carcinogenesis has been leveraged in an attempt to identify markers of disease and treatment response. A study by Kim et al. investigated whether inflammatory markers could serve as prognostic indicators in patients with advanced PDAC undergoing gemcitabine-based chemotherapy. They found that neutrophil-to-lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and C-reactive protein to albumin ratio were independent predictors of overall survival.80 Despite such efforts, no individual level or ratio has been found to be sufficiently sensitive and specific, and therefore, several scoring systems have been proposed.
A study by Nurmi et al. combined CRP and CA19-9 into a prognostic score and examined patients with resectable or borderline-resectable PDAC. They found that patients with CRP and CA19-9 below a cutoff value (3 mg/l and 3700 U/L, respectively) had a disease-specific survival time of 54 months compared with only 16 months in patients whose CRP and CA19-9 were above the cutoff values.81
Other scoring tools have been suggested. A modified Glasgow prognostic score has been proposed that incorporates albumin and CRP levels as prognostic markers.82 The systemic inflammatory index (SII) was found to be an independent negative predictor of overall survival and is calculated by multiplying neutrophil and monocyte counts, then dividing them by the lymphocyte count.83 Frigerio et al. found that, in patients who had liver-only synchronous metastases and underwent pancreatectomy after complete regression of the metastases, NLR and SII were associated with overall survival.46
While several of these inflammatory markers show some promise, data have been inconsistent, and large-scale studies, particularly in oligometastatic disease, are warranted to define their role in guiding treatment decisions.
Liquid Biopsy Modalities
Liquid biopsy has been increasingly utilized in CRC, breast, and lung cancers to monitor treatment efficacy, disease progression, and therapy resistance.84 Various methods of liquid biopsy have been described, including circulating tumor cells (CTCs), cell-free nucleic acid (cfDNA and cfRNA), and extracellular vesicles, such as exosomes.
Circulating tumor DNA (ctDNA) is a small subpopulation derived from cfDNA. A recent meta-analysis of liquid biopsy methods found that the use of ctDNA in the diagnosis of PDAC had a sensitivity of only 0.64.85 Early in PDAC progression, the rate of necrosis and apoptosis is low, and only one ctDNA molecule may be detected in 5 ml of plasma, which could account for this result. However, ctDNA may have a more substantial role in the detection and monitoring of advanced PDAC. In a study of patients with resectable localized PDAC, ctDNA detection in the preoperative setting was associated with poorer recurrence-free survival and overall survival.86 Uesato et al. evaluated PDAC patients with liver metastasis and found that patients with detectable ctDNA levels had worse overall survival. The presence of ctDNA also significantly correlated with a higher number of liver metastases, lung and/or peritoneal metastases, and higher Ca19-9 levels.87
Beyond just quantitative measurements of cfDNA, assessment of the mutational landscape also might be illuminating. Several studies have shown that levels of KRAS mutation in cfDNA correlated with radiographic tumor response to therapy in patients with mPDAC and predicted early recurrence following curative-intent resection.88 A recent study of 512 patients with PDAC found that ctDNA KRAS mutations were detected in 57% of patients, and the frequency of KRAS mutation differed depending on the metastatic organ. The KRAS mutation detection rate was significantly higher in patients with metastasis to the liver (78%) compared with lung (46%) and lymph nodes (60%).89 Although the sensitivity and specificity of such assays is still lacking, identification of such genetic mutations may reveal biologic subtypes that can help to guide operative decision-making. Further studies in oligometastatic PDAC should explore the impact of such mutations on prognosis and tumor behavior.90
Circulating tumor cells (CTCs) also have been studied in a variety of cancers, and in some cases their levels may indicate distant disease.91 Court et al. found that PDAC patients with occult metastatic disease had significantly more CTCs measured preoperatively compared with patients who had local disease only. They also found CTCs to be an independent predictor of recurrence-free survival after surgery.92
CLUSTER, a prospective longitudinal study, found that patients who received preoperative chemotherapy had significantly lower CTCs, and surgical resection of the tumor resulted in significant reduction of CTCs. Preoperative numbers of CTCs also were found to be predictors of early recurrence within 12 months from surgery.93
As we learn more about liquid biopsy techniques, findings should be correlated with current clinical practices (such as tumor markers and radiographic markers) to determine their clinical utility in following cancer progression and recurrence. Clarification on the role of liquid biopsy in PDAC management will create more datapoints for operative decision-making.
Conclusions
Metastasectomy for PDAC has increased in recent years, despite a lack of consensus definition for what constitutes oligometastatic disease. The majority of studies have focused on liver and lung metastases, with some exploration into peritoneal disease. The most common organ for metastatic spread and distant recurrence—the liver—also is the site associated with the worst prognosis compared with other sites of metastatic spread. In this population, data suggest that incorporating systemic chemotherapy before operative resection is associated with improved survival, emphasizing the importance of attempting to “downstage” the tumor. In patients with pulmonary metastases, several studies have reported longer overall survival times in patients with longer disease-free intervals.
Most of the data available are retrospective in nature, with significant variability in methodology and results reporting. Often, these investigations have been done in experienced academic centers, making the results difficult to generalize. More importantly, it is worth noting that there is a selection bias inherent to these studies; patients undergoing metastasectomy with subsequently increased overall survival likely had favorable tumor biology to begin with. Our review of the data shows that response to chemotherapy, normalization of biochemical markers, and longer disease-free intervals are associated with improved overall survival. This demonstrates that selection bias is not a flaw; it reveals a crucial detail about attempts to predict tumor behavior.
Current methods for estimating tumor biology are rudimentary, with varying levels of accuracy. At present, the best proxy for PDAC behavior is a response to chemotherapy, measured by some combination of trends in biochemical markers (such as Ca19-9) and radiographic findings or the rate of tumor growth and/or development of metastases. We now have under investigation newer modalities, such as liquid biopsy techniques, and investigations of the mutational landscape, which, although in their early stages, have shown promising results. As we gain a better understanding of the complexity of PDAC on a cellular and molecular level, we must move towards a personalized approach when selecting therapies. Investigating these individualized methods of predicting biologic behavior will enable a better understanding of those patients who would benefit from aggressive surgical approaches and metastasectomy.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17(55):603–5.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151(8):e161137.
Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4(7):963–9.
Mancini BR, Stein S, Lloyd S, et al. Chemoradiation after FOLFIRINOX for borderline resectable or locally advanced pancreatic cancer. J Gastrointest Oncol. 2018;9(6):982–8.
Kang MJ, Kim SW. Paradigm shift for defining the resectability of pancreatic cancer. Ann Hepatobiliary Pancreat Surg. 2021;25(4):451–5.
Tanaka M, Mihaljevic AL, Probst P, et al. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br J Surg. 2019;106(12):1590–601.
Sahin IH, Elias H, Chou JF, Capanu M, O’Reilly EM. Pancreatic adenocarcinoma: insights into patterns of recurrence and disease behavior. BMC Cancer. 2018;18(1):769.
Elias ML, Behbahani S, Maddukuri S, John AM, Schwartz RA, Lambert WC. Prolonged overall survival following metastasectomy in stage IV melanoma. J Eur Acad Dermatol Venereol. 2019;33(9):1719–25.
House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(5):744–52.
Lyon TD, Thompson RH, Shah PH, et al. Complete surgical metastasectomy of renal cell carcinoma in the post-cytokine era. J Urol. 2020;203(2):275–82.
Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14(12):3481–91.
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8–10.
Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One. 2011;6(12):e28650.
Joharatnam-Hogan N, Wilson W, Shiu KK, et al. Multimodal treatment in metastatic colorectal cancer (mCRC) improves outcomes-The University College London hospital (UCLH) experience. Cancers (Basel). 2020;12(12):3545.
Ottaiano A, Caraglia M, Di Mauro A, et al. Evolution of mutational landscape and tumor immune-microenvironment in liver oligo-metastatic colorectal cancer. Cancers (Basel). 2020;12(10):3073.
Rim CH, Cho WK, Lee JH, et al. Role of local treatment for oligometastasis: A comparability-based meta-analysis. Cancer Res Treat. 2022;54(4):953–69.
Cho HL, Balboni T, Christ SM, Turner B, Spektor A, Perni S. Is oligometastatic cancer curable? A survey of oncologist perspectives, decision making, and communication. Adv Radiat Oncol. 2023;8(5):101221.
Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–66.
Marshall JL. Managing potentially resectable metastatic colon cancer. Gastrointest Cancer Res. 2008;2(4 Suppl):S23–6.
Cloyd JM, Wiseman JT, Pawlik TM. Surgical management of pancreatic neuroendocrine liver metastases. J Gastrointest Oncol. 2020;11(3):590–600.
Tyrell R, Antia C, Stanley S, Deutsch GB. Surgical resection of metastatic melanoma in the era of immunotherapy and targeted therapy. Melanoma Manag. 2017;4(1):61–8.
Ollila DW, Gleisner AL, Hsueh EC. Rationale for complete metastasectomy in patients with stage IV metastatic melanoma. J Surg Oncol. 2011;104(4):420–4.
Le Large TYS, Bijlsma MF, Kazemier G, van Laarhoven HWM, Giovannetti E, Jimenez CR. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin Cancer Biol. 2017;44:153–69.
Gemenetzis G, Groot VP, Blair AB, et al. Incidence and risk factors for abdominal occult metastatic disease in patients with pancreatic adenocarcinoma. J Surg Oncol. 2018;118(8):1277–84.
Liu Q, Zhang R, Michalski CW, Liu B, Liao Q, Kleeff J. Surgery for synchronous and metachronous single-organ metastasis of pancreatic cancer: a SEER database analysis and systematic literature review. Sci Rep. 2020;10(1):4444.
Zhong Y, Macgregor-Das A, Saunders T, et al. Mutant p53 together with TGFbeta signaling influence organ-specific hematogenous colonization patterns of pancreatic cancer. Clin Cancer Res. 2017;23(6):1607–20.
Wright GP, Poruk KE, Zenati MS, et al. Primary tumor resection following favorable response to systemic chemotherapy in stage IV pancreatic adenocarcinoma with synchronous metastases: a bi-institutional analysis. J Gastrointest Surg. 2016;20(11):1830–5.
Okusaka T, Ikeda M, Fukutomi A, et al. Phase II study of FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105(10):1321–6.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Frigerio I, Regi P, Giardino A, et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol. 2017;24(8):2397–403.
Nagai M, Wright MJ, Ding D, et al. Oncologic resection of pancreatic cancer with isolated liver metastasis: favorable outcomes in select patients. J Hepatobiliary Pancreat Sci. 2023. https://doi.org/10.1002/jhbp.1303.
Horn SR, Stoltzfus KC, Lehrer EJ, et al. Epidemiology of liver metastases. Cancer Epidemiol. 2020;67:101760.
Groot VP, Gemenetzis G, Blair AB, et al. Implications of the pattern of disease recurrence on survival following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(8):2475–83.
Bachellier P, Addeo P, Averous G, Dufour P. Resection of pancreatic adenocarcinomas with synchronous liver metastases: a retrospective study of prognostic factors for survival. Surgery. 2022;172(4):1245–50.
Safi SA, Fluegen G, Rehders A, et al. Surgical margin clearance and extended chemotherapy defines survival for synchronous oligometastatic liver lesions of the ductal adenocarcinoma of the pancreas. Int J Clin Oncol. 2021;26(10):1911–21.
Shao Y, Feng J, Hu Z, et al. Feasibility of pancreaticoduodenectomy with synchronous liver metastasectomy for oligometastatic pancreatic ductal adenocarcinoma: a case-control study. Ann Med Surg (Lond). 2021;62:490–4.
Yang J, Zhang J, Lui W, et al. Patients with hepatic oligometastatic pancreatic body/tail ductal adenocarcinoma may benefit from synchronous resection. HPB (Oxford). 2020;22(1):91–101.
Andreou A, Knitter S, Klein F, et al. The role of hepatectomy for synchronous liver metastases from pancreatic adenocarcinoma. Surg Oncol. 2018;27(4):688–94.
Hackert T, Niesen W, Hinz U, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43(2):358–63.
Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160(1):136–44.
Shi HJ, Jin C, Fu DL. Preoperative evaluation of pancreatic ductal adenocarcinoma with synchronous liver metastasis: diagnosis and assessment of unresectability. World J Gastroenterol. 2016;22(45):10024–37.
Schwarz C, Fitschek F, Primavesi F, et al. Metachronous hepatic resection for liver only pancreatic metastases. Surg Oncol. 2020;35:169–73.
Kandel P, Wallace MB, Stauffer J, et al. Survival of patients with oligometastatic pancreatic ductal adenocarcinoma treated with combined modality treatment including surgical resection: a pilot study. J Pancreat Cancer. 2018;4(1):88–94.
Frigerio I, Malleo G, de Pastena M, et al. Prognostic factors after pancreatectomy for pancreatic cancer initially metastatic to the liver. Ann Surg Oncol. 2022;29(13):8503–10.
Wei M, Shi S, Hua J, Xu J, Yu X, Chinese Study Group for Pancreatic C. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: Protocol of a multicentre, prospective, randomised phase III control trial (CSPAC-1). BMJ Open. 2019;9(12):e033452.
Gebauer F, Damanakis AI, Popp F, et al. Study protocol of an open-label, single arm phase II trial investigating the efficacy, safety and quality of life of neoadjuvant chemotherapy with liposomal irinotecan combined with Oxaliplatin and 5-fluorouracil/Folinic acid followed by curative surgical resection in patients with hepatic Oligometastatic adenocarcinoma of the pancreas (HOLIPANC). BMC Cancer. 2021;21(1):1239.
Zanini N, Lombardi R, Masetti M, Giordano M, Landolfo G, Jovine E. Surgery for isolated liver metastases from pancreatic cancer. Updates Surg. 2015;67(1):19–25.
Mitsuka Y, Yamazaki S, Yoshida N, Yan M, Higaki T, Takayama T. Time interval-based indication for liver resection of metastasis from pancreatic cancer. World J Surg Oncol. 2020;18(1):294.
Vassos N, Piso P. Metastatic colorectal cancer to the peritoneum: Current treatment options. Curr Treat Options Oncol. 2018;19(10):49.
Goere D, Malka D, Tzanis D, et al. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257(6):1065–71.
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43.
Yamada S, Fujii T, Yamamoto T, et al. Conversion surgery in patients with pancreatic cancer and peritoneal metastasis. J Gastrointest Oncol. 2021;12(Suppl 1):S110–7.
Yamamoto T, Satoi S, Yamaki S, et al. Intraperitoneal paclitaxel treatment for patients with pancreatic ductal adenocarcinoma with peritoneal dissemination provides a survival benefit. Cancers (Basel). 2022;14(5):1354.
Di Giorgio A, Sgarbura O, Rotolo S, et al. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Ther Adv Med Oncol. 2020;12:1758835920940887.
Rotolo S, Ferracci F, Santullo F, et al. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): a case report of a multimodal treatment for peritoneal metastases of pancreatic origin. Int J Surg Case Rep. 2020;77S(Suppl):S75–8.
Groot VP, Blair AB, Gemenetzis G, et al. Isolated pulmonary recurrence after resection of pancreatic cancer: the effect of patient factors and treatment modalities on survival. HPB (Oxford). 2019;21(8):998–1008.
Homma Y, Endo I, Matsuyama R, et al. Outcomes of lung metastasis from pancreatic cancer: a nationwide multicenter analysis. J Hepatobiliary Pancreat Sci. 2022;29(5):552–61.
Yasukawa M, Kawaguchi T, Kawai N, Tojo T, Taniguchi S. Surgical treatment for pulmonary metastasis of pancreatic ductal adenocarcinoma: study of 12 cases. Anticancer Res. 2017;37(10):5573–6.
Shimizu T, Taniguchi K, Asakuma M, et al. Initial pulmonary metastasis after pancreatectomy for pancreatic ductal adenocarcinoma. Surg Today. 2020;50(4):413–8.
Kaiho T, Suzuki H, Yamamoto T, et al. Surgical outcomes of pulmonary metastasis from hepatopancreatobiliary carcinomas: a comparison with pulmonary metastasis from colorectal carcinomas. Surg Today. 2019;49(9):762–8.
Ilmer M, Schiergens TS, Renz BW, et al. Oligometastatic pulmonary metastasis in pancreatic cancer patients: Safety and outcome of resection. Surg Oncol. 2019;31:16–21.
Robinson LA, Tanvetyanon T, Springett G, et al. Pulmonary metastasectomy for suspected pancreaticobiliary cancer. J Thorac Cardiovasc Surg. 2016;152(1):75–82.
Yun WG, Kwon W, Han Y, et al. Can surgical resection of metastatic lesions be beneficial to pancreatic ductal adenocarcinoma patients with isolated lung metastasis? Cancers (Basel). 2022;14(9):2067.
Nakajima M, Ueno T, Suzuki N, et al. Novel indications for surgical resection of metachronous lung metastases from pancreatic cancer after curative resection. J Clin Gastroenterol. 2017;51(5):e34–8.
Klein F, Puhl G, Guckelberger O, et al. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract. 2012;2012:939350.
Ren W, Xourafas D, Ashley SW, Clancy TE. Temporal assessment of prognostic factors in patients with pancreatic ductal adenocarcinoma undergoing neoadjuvant treatment and resection. J Surg Res. 2021;257:605–15.
Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol. 2016;42(10):1533–9.
Hua YQ, Wang P, Zhu XY, et al. Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: an analysis of safety and efficacy. Pancreatology. 2017;17(6):967–73.
Satoi S, Yamamoto T, Yamaki S, Sakaguchi T, Sekimoto M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020;4(1):6–13.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S-50S.
Panda A, Garg I, Truty MJ, et al. Borderline resectable and locally advanced pancreatic cancer: FDG PET/MRI and CT tumor metrics for assessment of pathologic response to neoadjuvant therapy and prediction of survival. AJR Am J Roentgenol. 2021;217(3):730–40.
Abdelrahman AM, Goenka AH, Alva-Ruiz R, et al. FDG-PET Predicts neoadjuvant therapy response and survival in borderline resectable/locally advanced pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2022;20(9):1023–32.
Takeda T, Sasaki T, Okamoto T, et al. Outcomes of pancreatic cancer with liver oligometastasis. J Hepatobiliary Pancreat Sci. 2023;30(2):229–39.
Grollman EF, Kobata A, Ginsburg V. An enzymatic basis for Lewis blood types in man. J Clin Invest. 1969;48(8):1489–94.
Azizian A, Ruhlmann F, Krause T, et al. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10(1):1332.
Lee KJ, Yi SW, Chung MJ, et al. Serum CA 19–9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J. 2013;54(3):643–9.
Hank T, Klaiber U, Hinz U, et al. Oncological outcome of conversion surgery after preoperative chemotherapy for metastatic pancreatic cancer. Ann Surg. 2022;277(5):e1089–98.
Kim HJ, Lee SY, Kim DS, et al. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J Intern Med. 2020;35(1):171–84.
Nurmi AM, Mustonen HK, Stenman UH, Seppanen HE, Haglund CH. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci Rep. 2021;11(1):781.
Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow inflammation outcome study. Br J Cancer. 2011;104(4):726–34.
Murthy P, Zenati MS, Al Abbas AI, et al. Prognostic value of the systemic immune-inflammation index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol. 2020;27(3):898–906.
Hench IB, Hench J, Tolnay M. Liquid biopsy in clinical management of breast, lung, and colorectal cancer. Front Med (Lausanne). 2018;5:9.
Zhu Y, Zhang H, Chen N, Hao J, Jin H, Ma X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99(3):e18581.
Lee B, Lipton L, Cohen J, et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol. 2019;30(9):1472–8.
Uesato Y, Sasahira N, Ozaka M, Sasaki T, Takatsuki M, Zembutsu H. Evaluation of circulating tumor DNA as a biomarker in pancreatic cancer with liver metastasis. PLoS One. 2020;15(7):e0235623.
Perets R, Greenberg O, Shentzer T, et al. Mutant KRAS circulating tumor DNA is an accurate tool for pancreatic cancer monitoring. Oncologist. 2018;23(5):566–72.
Umemoto K, Sunakawa Y, Ueno M, et al. Clinical significance of circulating-tumour DNA analysis by metastatic sites in pancreatic cancer. Br J Cancer. 2023;128(8):1603–8.
Margonis GA, Kreis ME, Wolfgang CL, Weiss MJ. Mutation status and surgical selection. J Surg Oncol. 2019;119(5):616–22.
Jin KT, Chen XY, Lan HR, et al. Current progress in the clinical use of circulating tumor cells as prognostic biomarkers. Cancer Cytopathol. 2019;127(12):739–49.
Court CM, Ankeny JS, Sho S, et al. Circulating tumor cells predict occult metastatic disease and prognosis in pancreatic cancer. Ann Surg Oncol. 2018;25(4):1000–8.
Gemenetzis G, Groot VP, Yu J, et al. Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: Results of the prospective CLUSTER study. Ann Surg. 2018;268(3):408–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None. The authors have no commercial interest in the subject of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koti, S., Demyan, L., Deutsch, G. et al. Surgery for Oligometastatic Pancreatic Cancer: Defining Biologic Resectability. Ann Surg Oncol 31, 4031–4041 (2024). https://doi.org/10.1245/s10434-024-15129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15129-8