Abstract
Background
The multimodality management of patients with gastroesophageal cancers is rapidly evolving, with the introduction of new therapies against potential molecular targets paving the way to personalized medicine for patients with both resectable and metastatic disease. Over the past 2 years, several important studies evaluating these new targeted therapies, as well as minimally invasive surgical approaches to gastric cancer, have been published.
Methods
This review article summarizes the top studies published in gastric cancer over the past 2 years that are fundamentally changing our practice approach to gastric cancer patients.
Results
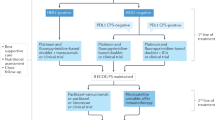
First, the long-term safety and efficacy of laparoscopic distal gastrectomy as compared with open gastrectomy for locally advanced gastric cancer was confirmed with the publication of the 5-year outcomes of the CLASS-01 and KLASS-02 randomized clinical trials. In addition, several important studies of perioperative immunotherapy for patients with resectable gastric or gastroesophageal junction cancers are ongoing, and in 2022, an interim analysis of the DANTE trial and the final results of the GERCOR NEONIPIGA study were reported. Lastly, the KEYNOTE-859 and SPOTLIGHT trials address an unmet need for additional targeted therapies for patients with previously untreated, human epidermal growth factor receptor-2 (HER2)-negative, unresectable or metastatic gastroesophageal cancers, incorporating immune checkpoint inhibitors and targeting Claudin-18 isoform 2 (CLDN18.2) with the monoclonal antibody zolbetuximab, respectively.
Conclusions
This article summarizes the findings and implications of several important studies published over the past 2 years that are fundamentally changing the way we treat patients with gastroesophageal cancer.
Similar content being viewed by others
References
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Kim HH, Han SU, Kim MC, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–13.
Son SY, Hur H, Hyung WJ, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: 5-year outcomes of the KLASS-02 randomized clinical trial. JAMA Surg. 2022;157:879–86.
Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321:1983–92.
Huang C, Liu H, Hu Y, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg. 2022;157:9–17.
van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic versus open gastrectomy for gastric cancer (LOGICA): a multicenter randomized clinical trial. J Clin Oncol. 2021;39:978–89.
Pietrantonio F, Miceli R, Raimondi A, et al. Individual patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin Oncol. 2019;37:3392–400.
André T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J Clin Oncol. 2023;41:255–65.
Raimondi A, Palermo F, Prisciandaro M, et al. TremelImumab and durvalumab combination for the non-operative management (NOM) of microsatellite InstabiliTY (MSI)-high resectable gastric or gastroesophageal junction cancer: the multicentre, single-arm, multi-cohort, phase II INFINITY study. Cancers (Basel). 2021;13:2839.
Lorenzen S, Götze TO, Thuss-Patience P, et al. Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 Trial. J Clin Oncol. 2023; JCO2300975.
Janjigian YY, Van Cutsem E, Muro K, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol. 2022;18:2465–73.
Bang YJ, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019;15:943–52.
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40.
Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47.
Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 Phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–80.
Fuchs CS, Dwoi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial [published correction appears in JAMA Oncol. 2019 Apr 1;5:579]. JAMA Oncol. 2018;4:e180013.
Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–33.
Rha SY, Oh DY, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–95.
Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402:2197–208.
Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401:1655–68.
Klempner SJ, Lee KW, Shitara K, et al. ILUSTRO: phase II multicohort trial of zolbetuximab in patients with advanced or metastatic claudin 18.2-positive gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2023;29:3882–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
John T. Mullen has no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mullen, J.T. Top Gastric Cancer Articles from 2022 and 2023 to Inform Your Cancer Practice. Ann Surg Oncol 31, 3978–3983 (2024). https://doi.org/10.1245/s10434-024-15072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15072-8