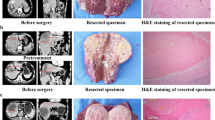
Abstract
Background
Combination treatment with transcatheter arterial chemoembolization (TACE), lenvatinib, and anti-programmed death-1 (anti-PD-1) antibodies (triple therapy) has a high rate of tumor response and converted resection for initially unresectable hepatocellular carcinoma (uHCC) patients. This study aimed to assess the outcomes of salvage surgery in uHCC patients after conversion therapy with triple therapy.
Methods
uHCC patients who met the criteria for hepatectomy after receiving triple therapy as first-line treatment were eligible for inclusion in this study. The overall survival (OS) and progression-free survival (PFS) rates in patients who received salvage surgery (SR group) and those who did not (non-SR group) were compared.
Results
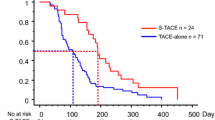
Of the 144 patients assessed, 91 patients underwent salvage surgery and 53 did not. The OS rates in the SR group were significantly better than those in the non-SR group. The 1- and 2-year OS rates in the SR group were 92.0% and 79.9%, respectively, whereas those in the non-SR group were 85.5% and 39.6 %, respectively (p = 0.007); however, there was no significant difference in the PFS rates. Upon further stratification, OS and PFS were significantly better in the SR group than in the non-SR group in patients who were assessed as partial responses (PR), while there was no significant difference in patients who were assessed as complete response (CR).
Conclusions
Salvage surgery is recommended and is associated with a favorable prognosis for uHCC patients who were assessed as PR after conversion therapy, however it may not be necessary for uHCC if CR was achieved.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440–51. https://doi.org/10.1002/hep.27745.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50. https://doi.org/10.1002/hep.29913.
Akaoka M, Haruki K, Taniai T, et al. Clinical significance of cachexia index in patients with hepatocellular carcinoma after hepatic resection. Surg Oncol. 2022;45:101881. https://doi.org/10.1016/j.suronc.2022.101881.
Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35:2155–66. https://doi.org/10.1111/liv.12818.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62. https://doi.org/10.1111/liv.12818.
Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11:227–52. https://doi.org/10.21037/hbsn-21-328.
Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. https://doi.org/10.1001/jamaoncol.2020.4564.
Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: upfront systemic therapy followed by curative conversion. Liver Cancer. 2021;10:539–44. https://doi.org/10.1159/000519749.
Cao F, Yang Y, Si T, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: a multicenter retrospective study. Front Oncol. 2021;11:783480. https://doi.org/10.3389/fonc.2021.783480.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. https://doi.org/10.1056/NEJMoa1915745.
Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. https://doi.org/10.1053/jhep.2002.33156.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–70. https://doi.org/10.1200/JCO.20.00808.
Zhu XD, Huang C, Shen YH, et al. Hepatectomy after conversion therapy using tyrosine kinase inhibitors plus anti-pd-1 antibody therapy for patients with unresectable hepatocellular carcinoma. Ann Surg Oncol. 2023;30:2782–90. https://doi.org/10.1245/s10434-022-12530-z.
Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? ten years of experience. Oncologist. 2016;21:1442–9. https://doi.org/10.1634/theoncologist.2016-0094.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–42. https://doi.org/10.1053/jhep.2003.50047.
Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15:155–60. https://doi.org/10.5582/bst.2021.01091.
Wu JY, Zhang ZB, Zhou JY, et al. Outcomes of salvage surgery for initially unresectable hepatocellular carcinoma converted by transcatheter arterial chemoembolization combined with lenvatinib plus anti-pd-1 antibodies: a multicenter retrospective study. Liver Cancer. 2022;12(3):229–37. https://doi.org/10.1159/000528356.
Qu WF, Ding ZB, Qu XD, et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real-world study. BJS Open. 2022;6:zrac114. https://doi.org/10.1093/bjsopen/zrac114.
Luo J, Peng ZW, Guo RP, et al. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259:286–95. https://doi.org/10.1148/radiol.10101072.
Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9:682–720. https://doi.org/10.1159/000509424.
Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the international study group of liver surgery (ISGLS). Surgery. 2011;149:713–24. https://doi.org/10.1016/j.surg.2010.10.001.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. https://doi.org/10.1016/j.surg.2010.10.001.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. https://doi.org/10.1038/s41572-020-00240-3.
Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–73. https://doi.org/10.1016/j.jhep.2021.11.030.
Fu Z, Li X, Zhong J, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663–75. https://doi.org/10.1007/s12072-021-10184-9.
Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-pd-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–40. https://doi.org/10.2147/JHC.S332420.
El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502. https://doi.org/10.1016/S0140-6736(17)31046-2.
Feun LG, Li YY, Wu C, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer. 2019;125:3603–14. https://doi.org/10.1002/cncr.32339.
Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. https://doi.org/10.3389/fimmu.2020.598877.
Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–72. https://doi.org/10.1038/s41571-021-00573-2.
Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8:299–311. https://doi.org/10.1159/000502905.
Masatoshi K, Tomoko A, Kazuomi U, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer. 2023;12(4):321–38. https://doi.org/10.1159/000529574.
Acknowledgment
This research was sponsored by the key Clinical Specialty Discipline Construction Program of Fujian, People’s Republic of China. The authors wish to thank all the staff of the participating hospitals for their kind cooperation and support, as well as all patients for their participation.
Funding
This research was supported by the Medical Innovation Project of Fujian Province (Grant Number: 2022CXA002), the Natural Science Foundation of Fujian Province, China (Grant Number: 2020J011105, 2022J011021), and the Startup Fund for Scientific Research, Fujian Medical University (Grant Number: 2018QH1112).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jun-Yi Wu, Jia-Yi Wu, Yang-Kai Fu, Xiang-Ye Ou, Shu-Qun Li, Zhi-Bo Zhang, Jian-Yin Zhou, Bin Li, Shuang-Jia Wang, Yu-Feng Chen, and Mao-Lin Yan have no related conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, JY., Wu, JY., Fu, YK. et al. Outcomes of Salvage Surgery Versus Non-Salvage Surgery for Initially Unresectable Hepatocellular Carcinoma After Conversion Therapy with Transcatheter Arterial Chemoembolization Combined with Lenvatinib Plus Anti-PD-1 Antibody: A Multicenter Retrospective Study. Ann Surg Oncol 31, 3073–3083 (2024). https://doi.org/10.1245/s10434-024-14944-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-14944-3