Abstract
Introduction
We sought to define the individual contributions of patient characteristics (PCs), hospital characteristics (HCs), case volume (CV), and social determinants of health (SDoH) on in-hospital mortality (IHM) after complex cancer surgery.
Methods
The California Department of Health Care Access and Information database identified patients who underwent esophagectomy (ES), pneumonectomy (PN), pancreatectomy (PD), or proctectomy (PR) for a malignant diagnosis between 2010 and 2020. Multi-level multivariable regression was performed to assess the proportion of variance explained by PCs, HCs, CV and SDoH on IHM.
Results
A total of 52,838 patients underwent cancer surgery (ES: n = 2,700, 5.1%; PN: n = 30,822, 58.3%; PD: n = 7530, 14.3%; PR: n = 11,786, 22.3%) across 294 hospitals. The IHM for the overall cohort was 1.7% and varied from 4.4% for ES to 0.8% for PR. On multivariable regression, PCs contributed the most to the variance in IHM (overall: 32.0%; ES: 21.6%; PN: 28.0%; PD: 20.3%; PR: 39.9%). Among the overall cohort, CV contributed 2.4%, HCs contributed 1.3%, and SDoH contributed 1.2% to the variation in IHM. CV was the second highest contributor to IHM among ES (5.3%), PN (5.3%), and PD (5.9%); however, HCs were a more important contributor among patients who underwent PR (8.0%). The unexplained variance in IHM was highest among ES (72.4%), followed by the PD (67.5%) and PN (64.6%) patient groups.
Conclusions
PCs are the greatest underlying contributor to variations in IHM following cancer surgery. These data highlight the need to focus on optimizing patients and exploring unexplained sources of IHM to improve quality of surgical care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Receipt of high-quality healthcare is hindered by a growing and diverse set of barriers. Potential factors including patient characteristics (PCs; i.e., age, sex, race), hospital characteristics (HCs; i.e., teaching status, nurse-patient ratio), social determinants (i.e., insurance coverage, travel time, social vulnerability index [SVI]) and hospital case volume (CV) have been identified as obstacles to optimal surgical care.1,2,3,4,5 In recent years, there has been a significant focus on the centralization of complex cancer operations in order to improve patient cancer outcomes.6
The volume-outcome relationship relating to complex surgical procedures has been extensively studied, with high-volume centers demonstrating lower overall morbidity and mortality.7,8,9,10 Some proponents of regionalization have advocated for the introduction of minimum-volume requirements, which would prohibit hospitals that do not meet the specified threshold from performing high-risk surgical procedures.11 Several healthcare systems have committed to implementing such a policy. However, other healthcare networks have been hesitant to embrace a ‘volume pledge’ due to concerns about potential trade-offs between improved outcomes and restricted access to surgical care.12 Contemporary studies examining the impact of hospital volume on major surgeries have yielded inconsistent findings relative to postoperative outcomes.12,13,14,15 A recent systematic review highlighted significant methodological disparities, including variations in how hospitals are categorized, statistical methods employed, and covariates considered that may influence the volume-outcome relationship.16 Furthermore, system-wide enhancements in surgical care have reduced surgical mortality over time, attenuating the impact of CV and leading to lower thresholds.17,18 Other concerns related to regionalization, such as exacerbation of existing socioeconomic and geospatial disparities, additional travel burden, and fragmentation of surgical care have also been raised.10 Hospital volume does not exist in isolation, and there is limited understanding of the broader context and environment in which a person resides. Specifically, the accessibility of essential resources such as food, water, lodging, transportation, employment, and education can vary dramatically according to one’s place of residence.19 In turn, these social determinants of health (SDoH) play a significant role in influencing access to high-quality healthcare.19,20
As complex cancer surgery becomes more centralized, it is important to examine the volume-outcome relationship through a more holistic lens that considers not only the surrounding SDoH but also individual-level patient factors and hospital structural characteristics. As such, the current study sought to investigate and quantify the role of PCs, HCs, CV, and SDoH on in-hospital mortality (IHM) after high-risk surgical procedures. We hypothesized that hospital volume would not be the most important contributor to the variation in IHM among patients undergoing complex oncologic surgery.
Methods
Data Source and Study Population
The California Department of Health Care Access and Information (HCAI) hospital discharge database was utilized to extract data on patients undergoing high-risk oncologic surgery between 2010 and 2020. As a department within the California Health and Human Services Agency, the HCAI is responsible for collecting and distributing healthcare information from licensed healthcare providers and hospitals in California, thereby capturing all hospital stays for patients in the state of California.10 Data were de-identified with encrypted ID assignments. Demographic data on the SVI and hospital-level data were obtained from the Centers for Disease Control and Prevention (CDC) SVI and American Hospital Association Survey databases, and subsequently linked using county FIPS codes and the AHAID identifier, respectively.21,22 The study was approved and informed consent for de-identified data was waived by the Institutional Review Board of the Ohio State University and the California Committee for the Protection of Human Subjects.
Individuals who underwent a complex oncologic procedure were defined as patients who had an elective resection for esophageal, lung, pancreatic, or rectal malignancies, identified using the International Classification of Diseases, Ninth and Tenth Revision diagnosis and procedure codes (see the Appendix).4,10 High-volume hospitals were identified based on established criterion from the Leapfrog Group, which recognizes eight critical procedures with a strong relationship between CV and outcomes.23 In particular, the minimum procedural volume thresholds for esophageal, lung, pancreatic, and rectal resections were set at 20, 40, 20, and 16, respectively.23
Primary Exposures and Outcome Interest
Variables included from the California HCAI, AHA and CDC SVI dataset included the following.
-
(1)
PCs: Age, sex, race/ethnicity, type of cancer surgery, and Elixhauser comorbidities.
-
(2)
HCs: American College of Surgeons (ACS) cancer program accreditation status, teaching status, affiliated medical school, total hospital, and intensive care unit (ICU) beds, total number of operating rooms, and staffing (physician full-time equivalents/bed). Other variables such as equipment available (i.e., interventional radiology, endoscopic retrograde cholangiopancreatography), nurse-patient ratio, trauma facilities and bed size were extracted but were excluded from the analyses due to statistical insignificance.
-
(3)
CV: Mean annual hospital CV was computed as a continuous variable to minimize the impact of annual fluctuations in hospital volume.9
-
(4)
SDoH: Insurance coverage, SVI, and real-world driving time.
The primary outcome of interest was patient IHM, calculated based on disposition at discharge, and the relative contributions of PCs, CV, HCs and SDoH to variations in IHM.
Geospatial Analysis
Data were processed using ARCGIS Pro (Redlands, CA, USA: Environmental Systems Research Institute, Inc., 2010). Hospitals were geocoded using the reported address in the HCAI database. Network analysis was conducted to evaluate real-world travel distance and time from the centroid of the ZIP code of each patient to the hospital they underwent surgery at using the origin destination (OD) cost matrix function within ARCGIS Pro.24 In addition to distance traveled, real-world driving time was utilized as it more accurately reflects the actual impact of travel on patients, particularly in urban and rural areas where driving times may vary greatly.25
Risk- and Reliability-Adjusted Hospital Mortality Groups
To ensure fair comparison among hospitals, IHM rates were reliability-adjusted, ordered based on increasing IHM rates, and subsequently divided into tertiles (low: <33rd percentile; medium: 33rd to 66th percentile; and high: > 66th percentile) based on a priori cut-offs to allow for adequate power in the analyses.26,27 Reliability adjustment was used to reduce statistical ‘noise’ to allow for more accurate comparisons between facilities reporting risk-adjusted hospital outcomes.27 Mortality groups were calculated for each individual operation (i.e., esophagectomy [ES], pneumonectomy [PN], pancreatectomy [PD], and proctectomy [PR]).
Statistical Analysis
Continuous variables were reported as median with interquartile range (IQR; i.e., PCs) or means with standard deviations (i.e., HCs), and discrete variables were reported as frequencies with percentages. Univariable comparisons were performed using the t-test for continuous variables and Chi-square test for categorical variables. Multi-level mixed-effect logistic regression models were constructed for the overall cohort and each individual procedure to determine the factors associated with IHM, as well as the proportion of variance explained. Four models were constructed that iteratively added (1) PCs; (2) CV; (3) HCs; and (4) SDoH with random intercepts for hospitals to account for clustering to calculate the percentage of variance in IHM explained by the addition of the variables in the model. All models were controlled for age, sex, insurance status, Elixhauser comorbidities, year of diagnosis, cancer type (for overall model), HCs, and social determinants. Sensitivity analyses were performed excluding the ES cohort and revealed no difference in outcomes. Inclusion of specific HCs was determined based on clinical knowledge. Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). All statistical analyses were derived from two-tailed tests and a p-value of <0.05 was considered statistically significant. The analyses were performed using STATA version 17.0 (StataCorp LLC, College Station, TX, USA).
Results
Baseline Characteristics of Patients and Hospitals
A total of 52,838 patients underwent a complex oncologic operation (ES: n = 2700, 5.1%; PN: n = 30,822, 58.3%; PD: n = 7530, 14.3%; PR: n = 11,786, 22.3%) across 294 hospitals. Overall, median patient age was 68.0 years (60.0–75.0), one-half of patients were male (n = 26,526, 50.2%), and most patients were White (n = 33,327, 63.1%), and had Medicare (n = 30,345, 57.4%) or private (n = 16,357, 31.0%) insurance. Smaller subgroups of patients were Black (n = 2653, 5.0%), Hispanic (n = 7503, 14.2%), Asian (n = 7121, 13.5%) or other race/ethnicity (n = 2234, 4.2%). Most patients underwent surgery at a high-volume (n = 36,037, 68.2%) cancer-accredited center (n = 31,571, 59.8%), and traveled a median distance and time of 12.3 miles (5.5–25.8) for 20.0 min (11.8–35.4), respectively, to reach their surgical center of choice. Differences in PCs according to individual cancer procedure are noted in Table 1.
Among the 294 hospitals included, 19 (6.5%) were major teaching hospitals, 151 (51.4%) were affiliated with a medical school, and 108 (36.7%) were accredited cancer programs. The mean number of hospital beds per hospital was 178, mean number of operating rooms was 8 per hospital, and the mean physician full-time equivalent (FTE)/bed ratio was 0.1. There were 158 hospitals that performed ES, with a mean annual ES volume of four cases per hospital; 245 hospitals performed PN, with a mean annual PN volume of 29; 166 hospitals performed PD, with a mean annual PD volume of 11 cases per hospital; and 255 hospitals performed PR with a mean annual PR volume of 14 cases per hospital.
The IHM for the overall cohort was 1.7% (n = 899) and decreased significantly from 2.2% in 2010 to 1.2% in 2020 (p < 0.001). IHM varied according to cancer procedure, ranging from 4.4% for ES, 2.4% for PD, 1.7% for PN, and 0.8% for PR (p < 0.001). Patients who experienced IHM were more likely to be older (73.0 years vs. 68.0 years), have a greater median Elixhauser comorbidity index (5.0 vs. 2.0), were more frequently male (64.4% vs. 50.0%), had Medicare insurance (74.6% vs. 57.1%), and resided in areas with higher SVI (71.9 vs. 56.1) [all p < 0.001]. In contrast, patients treated at high-volume (68.6% vs. 47.4%), cancer-accredited (59.8% vs. 56.0%) or teaching (35.3% vs. 24.4%) hospitals, as well as patients with private insurance (31.2% vs. 14.2%) and who traveled longer distances (12.4 vs. 9.4 miles) and had longer travel times (20.1 vs. 16.9 min) were less likely to experience an IHM event (all p < 0.001) [Table 2].
Patient and Hospital Characteristics Across Reliability-Adjusted Mortality Groups
In assessing individual PCs across low, medium, and high mortality groups, patients from the ES subgroup were more likely to undergo surgery at a low-mortality hospital versus a high- or medium-mortality center (5.9% vs. 4.5% and 4.9%). Patients from the high-mortality group were more likely to be covered with Medicaid (9.9% vs. 9.2% and 6.6%), have a higher median Elixhauser comorbidity index (3.0 vs. 2.0 for both), and be of Hispanic (15.0% vs. 14.2% and 13.4%) or Asian (14.3% vs. 12.5% and 13.6%) race/ethnicity compared with the medium- and low-mortality groups. In contrast, patients in the low-mortality group were more likely to go to a high-volume (88.8% vs. 58.1% and 57.1%), cancer-accredited (64.6% vs. 62.1% and 52.4%) or teaching (50.5% vs. 34.1% and 20.5%) hospital, have private insurance (34.2% vs. 29.6% and 29.0%), and have greater median travel distances (15.1 vs. 11.1 and 11.3 miles) and times (24.1 vs. 18.6 and 17.9 min) versus patients with medium and high mortality (Table 1 in the electronic supplementary material [ESM]).
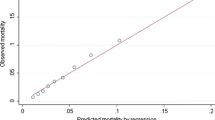
The variability in IHM across reliability-adjusted mortality rates relative to hospital CV is presented in Fig. 1. After classification into mortality groups, most hospitals fell into the high-mortality group for the ES (n = 90, 60.0%), PD (n = 92, 55.4%) and PR (n = 134, 52.5%) cohorts; however, most of the hospitals among the PN subgroup were more likely to belong to the medium mortality group (n = 146, 59.6%) [all p < 0.001]. Notably, hospitals in the lowest mortality group had the highest mean annual CV (ES: 11.1 ± 12.2; PN: 76.6 ± 66.6; PD: 45.2 ± 41.7; PR: 36.7 ± 25.7), mean number of hospital beds (ES: 380 ± 236; PN: 376 ± 236; PD: 292 ± 187; PR: 369 ± 246), and mean number of operating rooms (ES: 21 ± 14; PN: 18 ± 15; PD: 17 ± 12; PR: 21 ± 16) [all p < 0.001]. The variation in facility characteristics across hospital mortality groups is noted in ESM Table 2.
Multivariable Analysis and Proportion of Variance Explained
On multivariable logistic regression analysis, PCs, hospital volume, and social determinants were significantly associated with IHM. Notably, increasing age (1-year increase; OR 1.03, 95% CI 1.02–1.04), Hispanic (reference: White; OR 1.36, 95% CI 1.04–1.76) and Asian (reference: White; OR 1.35, 95% CI 1.00–1.82) race/ethnicity, greater Elixhauser comorbidity index (1 unit increase; OR 1.63, 95% CI 1.58–1.68), SVI (1 unit increase; OR 1.01, 95% CI 1.01–1.01), as well as Medicare (reference: private; OR 1.36, 95% CI 1.04–1.78) and Medicaid (reference: private; OR 2.00, 95% CI 1.31–3.04) insurance were associated with higher IHM (all p < 0.05). In contrast, female sex (reference: male; OR 0.66, 95% CI 0.56–0.78), cancer surgery type (reference: ES; PN: OR 0.31, 95% CI 0.23–0.44; PD: OR 0.45, 95% CI 0.33–0.61; PR: OR 0.22, 95% CI 0.15–0.32), higher mean hospital volume (10-procedure increase; OR 0.99, 95% CI 0.98–1.00), and further driving times (10 min increase; OR 0.95, 95% CI 0.93–0.98) were associated with lower IHM (all p < 0.05).
The distribution of variance explained by PCs, CV, HCs, SDoH, and unknown factors is illustrated in Fig. 2. PCs contributed the most to the variance in IHM among the overall cohort (32.0%), as well as each individual operation (ES: 21.6%; PN: 28.0%; PD: 20.3%; PR: 39.9%). Of note, CV contributed just 2.4%, HCs contributed 1.3%, and SDoH contributed 1.2% to the variation in IHM among the overall cohort. Interestingly, CV was the second highest contributor to IHM among ES (5.3%), PN (5.3%), and PD (5.9%); however, HCs were a more important contributor among patients who underwent PR (8.0%). SDoH contributed <2.0% to the variation in IHM for all cancer surgery subtypes except for PD (5.1%). The unexplained variance in IHM was highest among ES (72.4%) followed by the PD (67.5%), PN (64.6%), and PR (45.5%) subgroups, respectively (Table 3).
Discussion
The provision of high-quality surgical services for cancer patients is crucial to ensure equitable healthcare. Rapid advancements in healthcare infrastructure and technology have ensured that regionalization of complex oncologic surgical care into large, multidisciplinary centers is likely to persist. However, recent studies examining the volume-outcome relationship have yielded conflicting results and raised several concerns regarding substantial methodological disparities, reduction in surgical mortality over time that have led to attenuation of the impact of CV, and worsening inequities in access to care for vulnerable populations.6,8,9,16,17,18 Moreover, the role of SDoH relative to surgical outcomes has been increasingly recognized over the last decade.19,28,29 For instance, recent work from our own group has emphasized the importance of neighborhood characteristics and social vulnerability on operative outcomes.30,31 The effect of hospital volume on postoperative outcomes does not exist in a vacuum, and the impact of other patient, hospital, and neighborhood-level factors on this association remains relatively ill-defined. Therefore, the current work was important as we specifically quantified the role of PCs, HCs, CV, and SDoH on IHM after complex oncologic surgery. Of note, the current study demonstrated that PCs contribute most to IHM (ranging from 20.3% in pancreatic surgery to 39.9% in rectal surgery) after major cancer surgery in the state of California. CV accounted for 4.9–5.9%, HCs between 1.0 and 8.0%, and SDoH was responsible for 0.4–5.1% of the variability in IHM. Importantly, more than one-half of the variance in IHM after esophageal, lung and pancreatic surgery remained unexplained.
Despite advancements in the delivery of cancer care at the national level, there is still significant variation in patient health outcomes. The current study builds on previous work from Martins et al. that examined variations in hospital mortality rates among a 2006–2011 surgical cohort, by exploring the role of additional contributors such as social determinants alongside patient- and hospital-level characteristics among a high-risk cancer surgery cohort during a more recent time period (2010–2020).26 Similar to the previous study, we noted that patient-level factors were the single most important contributor to IHM across hospitals in California, thereby highlighting the importance of risk identification, stratification and mitigation to improve postoperative outcomes. While the urgency associated with many surgical procedures often limits the potential for substantial improvements in patient functional status and health before surgery, the rapid rise in utilization of neoadjuvant therapy among cancer patients may provide a window of opportunity. Even short-term behavioral modifications such as quitting smoking have been associated with improved healthcare outcomes. Other noteworthy approaches include patient prehabilitation, which involves the focused optimization of modifiable risk factors, both physical and psychological, prior to undergoing surgery.32 Prehabilitation has lead to better postoperative outcomes, including reduced morbidity, lower IHM, shorter hospital stays, enhanced patient quality of life, faster recovery times, and decreased need for additional interventions.32 Nonetheless, implementing prehabilitation on a larger scale poses challenges and requires coordination among patients, social services, stakeholders and healthcare organizations. The Strong for Surgery program, initially launched at the University of Washington in 2012 and subsequently integrated as an ACS Quality Program may serve as an archetype of a successful prehabilitation effort.33 Today, this program has been adopted by more than 200 hospitals in the United States and engages various stakeholders within the healthcare system, including patients themselves, to optimize patient readiness before surgery.33 Moreover, prehabilitation also plays a significant role in the Enhanced Recovery after Surgery (ERAS) programs, which have gained momentum in US hospitals over the last two decades and have consistently been associated with improved surgical outcomes.34,35,36
The current study also examined the impact of structural characteristics within hospitals on variation in IHM, particularly in the context of surgery for rectal cancer (8.0%). Hospitals with advanced clinical services are more capable in preventing the incidence of postoperative complications, as well as in managing them effectively, thereby reducing the ‘failure to rescue’ phenomenon that is associated with high IHM rates.37,38,39 HCs such as availability of equipment and advanced medical technology, the capacity and care models within ICUs, staffing models, teaching status, and easy accessibility to essential healthcare professionals such as intensivists, rapid response teams, nurse practitioners, and physician assistants play a pivotal role in preventing the occurrence of failure to rescue, as these factors help to identify hospitals that are better equipped to recognize and effectively manage severe postoperative complications.40,41 For instance, recent research has demonstrated that the presence of a dedicated cancer program within a hospital is a more reliable predictor of IHM than surgical volume or other hospital attributes.42 Although certain fundamental structural characteristics of hospitals may be deemed essential for performing high-risk operations, implementing and sustaining such changes at the individual hospital level can present considerable difficulties. Consequently, interim strategies, such as directing higher-risk patients to hospitals with greater resources, may be explored to enhance clinical outcomes.43
Another important consideration that has gained traction over recent years is the role of SDoH. Although it may be useful to compare patients based on factors such as education, transportation, and financial resources, these assessments do not provide a comprehensive understanding of the holistic ‘lived experience’ of individuals within a particular community. Healthcare research and policy efforts that consider this wider social and environmental context might prove more effective, as social determinants and non-medical social needs can exert a significant impact on an individual’s overall well-being.19,20,28 To this effect, social vulnerability functions as a measure of the resilience of a community to external stressors and has been identified as a possible root cause of health inequities.30,31 Moreover, patient travel patterns function as another key determinant that impact access to high-quality surgical care.10,44,45 Patients may seek treatment at designated cancer centers in the hope of realizing improved outcomes; however, delays in appropriate cancer treatment to reach such centers can be problematic and can even result in worse cancer outcomes.45,46,47,48,49 It is also imperative to acknowledge the role of patient preferences, a factor that is often underestimated by policymakers and stakeholders. A seminal study conducted by Finlayson et al. revealed that 45% of patients preferred to receive treatment at a local medical center, even when the risk of mortality following surgery was twice as high there versus a regional center with half the mortality risk that was further away.50 Similarly, most patients undergoing gastrectomy in the state of California chose to forgo evidence-based decision making in order to undergo surgical treatment at centers closer to their area of residence.51 Yet another study highlighted that 74% of patients faced considerable financial and insurance-related barriers to long-distance travel for healthcare.52 Nevertheless, most of these patients indicated a willingness to travel further if some of these barriers could be mitigated.52 The current work is in line with these studies and demonstrates a reduction in IHM among patients who traveled further and longer to undergo high-risk surgical care. Collectively, these studies underscore the importance of addressing barriers to ensure equitable access when implementing policies based on the volume-outcome association.
Results from the current work also raise doubts on the appropriateness of utilizing arbitrary hospital CV thresholds, as proposed by initiatives such as the Volume Pledge, to drive health quality improvement. If contemporary hospital volume criteria are applied, over 70% of hospitals would be disqualified from performing complex oncologic procedures such as esophageal, lung, pancreatic or rectal surgery.17,53 Additionally, strict implementation of volume thresholds to promote regionalization of high-risk surgical care may further exacerbate inequities in healthcare access and contribute to the fragmentation of care, particularly among disadvantaged populations.4,10,54 Notwithstanding the fact that hospital CV is aimed at improving healthcare outcomes by increasing the number of cases as a quantifiable quality improvement measure, the past two decades and significant advancements in surgical research have demonstrated that this approach fails to consider factors such as patient preferences, geospatial access barriers, and patterns of insurance referrals. While there is undeniable evidence linking CV to outcomes, too much attention has been placed on the actual number of cases, thereby neglecting a more holistic approach to identifying areas for improvement. A potentially more effective and equitable approach would concentrate on patient prehabilitation, improvement of hospital resources, and engagement of socioeconomically vulnerable communities. Moreover, it is crucial that unexplained sources of variance in IHM are promptly identified. These unknown sources are likely related to complex factors that are difficult to capture in healthcare databases, such as the implementation of continuous quality improvement (CQI) initiatives, a surgical center’s culture of safety, and the multifaceted interactions between patients’ comorbidities, surgical procedures, and postoperative outcomes. Investigating these underlying factors could be facilitated by analyzing data with more clinical granularity, utilizing advanced artificial language methodologies such as natural language processing and machine learning, and employing robust statistical models such as mediation analyses.55,56
There are several limitations to consider when interpreting the current study results. Although, the California database provided for 100% capture and comprehensive data gathering on travel for all patients having surgery at California-licensed facilities, the data were restricted to one state. As a result, these findings may not be generalizable to other states with different geographies. Travel distance and time were calculated using StreetMap Premium in ARCGIS Pro geographical software, and while this was more accurate than straight-line travel distance studies, it may not have fully reflected real-world traffic patterns as certain datapoints on time of day traveled could not be assessed. Moreover, patients who rely on public transportation, especially those with limited resources, may experience significantly longer travel times due to the availability and efficiency of such services. County-level data were used for the calculation of SVI, hence there might have been residual heterogeneity. Larger counties, for instance, might have had inhabitants with a wide range of socioeconomic vulnerability. The primary endpoint of the study was IHM, thereby restricting the findings from extrapolation to alternative outcomes such as 30- or 90-day mortality that may have different proportionate contributions to variations in outcomes. Due to the restrictions of the HCAI database, the current study was unable to examine disease stage or long-term outcomes such as disease-free survival. Furthermore, admissions for selected operations were assessed rather than diagnosis of incident malignancy. While a distinction between locally advanced, regional, or metastatic disease could not be made, most patients with metastatic illness would not have been given the option of a resection, particularly for pancreatic and esophageal cancer. Finally, some individuals with potentially operable cancer might not have undergone surgery and as such were left out of the study cohort.
Conclusion
The current study demonstrates that most of the variability in IHM following complex cancer surgery stems from patient-level factors rather than hospital CV, HCs or social determinants. Furthermore, over half the variation in IHM remains unexplained, especially among esophageal, lung and pancreatic cancer. These data highlight the need to focus on patient optimization and on risk reduction in addition to initiatives that are aimed at regionalization, structural quality improvement and social determinants. Moreover, there is a need to explore the reasons for the hitherto unexplained factors that underlie IHM to improve the quality of surgical care.
Data availability
The data for this study were obtained from the California Office of State-Wide Health Planning and Development (OSHPD) hospital discharge database. There are restrictions to the availability of this data, which is used under license for this study. Data can be accessed with permission from the California Department of Health Care Access and Information.
References
Mosadeghrad AM. Factors affecting medical service quality. Iran J Public Health. 2014;43(2):210–20.
Burke LG, Frakt AB, Khullar D, Orav EJ, Jha AK. Association between teaching status and mortality in US hospitals. JAMA. 2017;317(20):2105–13.
Friese CR, Lake ET, Aiken LH, Silber JH, Sochalski J. Hospital nurse practice environments and outcomes for surgical oncology patients. Health Serv Res. 2008;43(4):1145–63.
Munir MM, Endo Y, Alaimo L, Moazzam Z, Lima HA, Woldesenbet S, et al. Impact of community privilege on access to care among patients following complex cancer surgery. Ann Surg. 2023;278(6):e1250–8.
Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33.
Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: Implications for patient access to optimal care. J Clin Oncol. 2009;27(28):4671–8.
Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37.
Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777.
Birkmeyer JD, Siewers AE, Finlayson EVA, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37.
Munir MM, Endo Y, Woldesenbet S, Beane J, Dillhoff M, Ejaz A, et al. Variations in travel patterns affect regionalization of complex cancer surgery in California. Ann Surg Oncol. 2023;30(13):8044–53.
Hospitals Move to Limit Low-Volume Surgeries [cited 2023 May 3]. Available at: https://www.usnews.com/news/articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries
Schwartz DM, Fong ZV, Warshaw AL, Zinner MJ, Chang DC. The hidden consequences of the volume pledge: “no patient left behind”? Ann Surg. 2017;265(2):273–4.
Farjah F, Grau-Sepulveda MV, Gaissert H, Block M, Grogan E, Brown LM, et al. Volume pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38(30):3518–27.
Jacobs RC, Groth S, Farjah F, Wilson MA, Petersen LA, Massarweh NN. Potential impact of “take the volume pledge” on access and outcomes for gastrointestinal cancer surgery. Ann Surg. 2019;270(6):1079.
Wasif N, Etzioni DA, Habermann E, Mathur A, Chang YH. Correlation of proposed surgical volume standards for complex cancer surgery with hospital mortality. J Am Coll Surg. 2020;231(1):45-52.e4.
Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5(1):204.
Wasif N, Etzioni DA, Habermann EB, Mathur A, Pockaj BA, Gray RJ, et al. Does improved mortality at low- and medium-volume hospitals lead to attenuation of the volume to outcomes relationship for major visceral surgery? J Am Coll Surg. 2018;227(1):85-93.e9.
Jogerst KM, Chang YHH, Etzioni DA, Mathur AK, Habermann EB, Wasif N. Identifying the optimal case-volume threshold for pancreatectomy in contemporary practice. Am J Surg. 2022;223(2):318–24.
Singh GK, Daus GP, Allender M, Ramey CT, Martin EK, Perry C, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation. Int J Matern Child Health AIDS. 2017;6(2):139–64.
Adler NE, Glymour MM, Fielding J. Addressing social determinants of health and health inequalities. JAMA. 2016;316(16):1641–2.
AHA Annual Survey DatabaseTM | AHA Data [cited 2023 Sep 19]. Available at: https://www.ahadata.com/aha-annual-survey-database
CDC/ATSDR Social Vulnerability Index (SVI). 2023 [cited 2023 Sep 19]. Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
Leapfrog [cited 2022 Aug 12]. Available at: https://www.leapfroggroup.org/ratings-reports/leapfrog-hospital-survey-report-series-issued-2019
Find Closest Facilities (Ready To Use)—ArcGIS Pro | Documentation [cited 2023 Jul 2]. Available at: https://pro.arcgis.com/en/pro-app/latest/tool-reference/ready-to-use/itemdesc-findclosestfacilities.htm
Boscoe FP, Henry KA, Zdeb MS. A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof Geogr. 2012;64(2):188.
Martins RS, Chang YH, Etzioni D, Stucky CC, Cronin P, Wasif N. Understanding variation in in-hospital mortality after major surgery in the United States. Ann Surg. 2023;278(6):865–72.
Dimick JB, Ghaferi AA, Osborne NH, Ko CY, Hall BL. Reliability Adjustment for Reporting Hospital Outcomes With Surgery. Ann Surg. 2012;255(4):703.
Social Determinants of Health - Healthy People 2030 | health.gov [cited 2022 Nov 17]. Available at: https://health.gov/healthypeople/priority-areas/social-determinants-health
Arcaya MC, Tucker-Seeley RD, Kim R, Schnake-Mahl A, So M, Subramanian SV. Research on neighborhood effects on health in the United States: a systematic review of study characteristics. Soc Sci Med. 2016;168:16–29.
Azap RA, Paredes AZ, Diaz A, Hyer JM, Pawlik TM. The association of neighborhood social vulnerability with surgical textbook outcomes among patients undergoing hepatopancreatic surgery. Surgery. 2020;168(5):868–75.
Diaz A, Hyer JM, Tsilimigras D, Pawlik TM. The impact of social vulnerability subthemes on postoperative outcomes differs by racial/ethnic minority status. Am J Surg. 2022;223(2):353–9.
Santa Mina D, van Rooijen SJ, Minnella EM, Alibhai SMH, Brahmbhatt P, Dalton SO, et al. Multiphasic prehabilitation across the cancer continuum: a narrative review and conceptual framework. Front Oncol. 2020;10:598425.
The ACS Strong for Surgery program: Changing clinician and system behavior to optimize health before surgery. The Bulletin. 2019 [cited 2023 Sep 19]. Available at: https://bulletin.facs.org/2019/10/the-acs-strong-for-surgery-program-changing-clinician-and-system-behavior-to-optimize-health-before-surgery/
Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMM, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275(2):e299-306.
Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155(3):233–42.
Gillis C, Ljungqvist O, Carli F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth. 2022;128(3):434–48.
Billingsley KG, Morris AM, Dominitz JA, Matthews B, Dobie S, Barlow W, et al. Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: understanding the volume-outcome relationship. Arch Surg Chic Ill. 2007;142(1):23–31.
Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250(6):1029–34.
Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368–75.
Ward ST, Dimick JB, Zhang W, Campbell D, Ghaferi AA. Association between hospital staffing models and failure to rescue. Ann Surg. 2019;270(1):91–4.
Sheetz KH, Dimick JB, Ghaferi AA. Impact of hospital characteristics on failure to rescue following major surgery. Ann Surg. 2016;263(4):692–7.
Friese CR, Earle CC, Silber JH, Aiken LH. Hospital characteristics, clinical severity, and outcomes for surgical oncology patients. Surgery. 2010;147(5):602–9.
Zhang W, Ayanian JZ, Zaslavsky AM. Patient characteristics and hospital quality for colorectal cancer surgery. Int J Qual Health Care J Int Soc Qual Health Care. 2007;19(1):11–20.
Diaz A, Burns S, D’Souza D, Kneuertz P, Merritt R, Perry K, et al. Accessing surgical care for esophageal cancer: patient travel patterns to reach higher volume center. Dis Esophagus. 2020;33(7):doaa006.
Turrentine FE, Buckley PJ, Sohn MW, Williams MD. Travel time influences readmission risk: geospatial mapping of surgical readmissions. Am Surg. 2017;83(6):573–82.
Birkmeyer NJO, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the national cancer institute have better surgical outcomes? Cancer. 2005;103(3):435–41.
Mehta R, Tsilimigras DI, Paredes AZ, Sahara K, Dillhoff M, Cloyd JM, et al. Dedicated cancer centers are more likely to achieve a textbook outcome following hepatopancreatic surgery. Ann Surg Oncol. 2020;27(6):1889–97.
Paulson EC, Mitra N, Sonnad S, Armstrong K, Wirtalla C, Kelz RR, et al. National cancer institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. 2008;248(4):675.
Wasif N, Chang YH, Pockaj BA, Gray RJ, Mathur A, Etzioni D. Association of distance traveled for surgery with short- and long-term cancer outcomes. Ann Surg Oncol. 2016;23(11):3444–52.
Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37(2):204–9.
Alvino DML, Chang DC, Adler JT, Noorbakhsh A, Jin G, Mullen JT. How far are patients willing to travel for gastrectomy? Ann Surg. 2017;265(6):1172.
Resio BJ, Chiu AS, Hoag JR, Brown LB, White M, Omar A, et al. Motivators, barriers, and facilitators to traveling to the safest hospitals in the united states for complex cancer surgery. JAMA Netw Open. 2018;1(7):e184595.
Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373(15):1388–90.
Gani F, Azoulay D, Pawlik TM. Evaluating trends in the volume-outcomes relationship following liver surgery: does regionalization benefit all patients the same? J Gastrointest Surg. 2017;21(3):463–71.
Barber EL, Garg R, Persenaire C, Simon M. Natural language processing with machine learning to predict outcomes after ovarian cancer surgery. Gynecol Oncol. 2021;160(1):182–6.
Woldesenbet S, Munir MM, Pawlik TM. Invited commentary: mediation analysis and cancer screening, identifying areas to target to improve cancer disparities. J Surg Oncol. 2023;128(1):7–8.
Acknowledgments
None.
Funding
No sources of funding were used to assist in the preparation of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Muhammad Musaab Munir, Selamawit Woldesenbet, Yutaka Endo, Mary Dillhoff, Jordan Cloyd, Aslam Ejaz, and Timothy M. Pawlik have no conflicts of interest to declare in relation to this work. The authors have no disclaimers to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munir, M.M., Woldesenbet, S., Endo, Y. et al. Variation in Hospital Mortality After Complex Cancer Surgery: Patient, Volume, Hospital or Social Determinants?. Ann Surg Oncol 31, 2856–2866 (2024). https://doi.org/10.1245/s10434-023-14852-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14852-y